What does the stomata look like? Characteristics of the main ecological groups of plants. Leaf senescence and leaf fall
FABRICS. CLASSIFICATION OF FABRICS.
The organization of higher plants is based on the principle of cell specialization, which lies in the fact that each cell of the body does not perform all its inherent functions, but only some, but more fully and perfectly.
Fabrics- stable, naturally repeating complexes of cells, similar in origin, structure and adapted to perform one or more functions.
There are various classifications of fabrics, but they are all quite arbitrary.
Depending on the main function, several groups of plant tissues are distinguished.
1. educational fabrics, or meristems,- have the ability to divide and form all other tissues.
2. Covering tissues:
Primary;
Secondary;
Tertiary.
3. Main fabrics- make up most of the plant body. The following main fabrics are distinguished:
Assimilation (chlorophyll-bearing);
Stockers;
Airborne (aerenchyma);
Aquifers.
4. Mechanical fabrics(supporting, skeletal):
Collenchyma;
Sclerenchyma.
5. Conductive fabrics:
Xylem (wood) is an ascending tissue;
Phloem (phloem) is a tissue of descending flow.
6. Excretory tissues:
External:
Glandular hairs;
Hydathodes - water stomata;
Sunbirds;
Internal:
Excretory cells with essential oils, resins, tannins;
Multicellular receptacles for secretions, lacticifers.
Based on the ability of cells to divide, two types of tissues are distinguished: educational, or meristems, And permanent- integumentary, excretory, basic, mechanical, conductive.
The fabric is called simple, if all its cells are identical in shape and function (parenchyma, sclerenchyma, collenchyma). Complex tissues consist of cells that are different in shape, structure and function, but are related by a common origin (for example, xylem, phloem).
There is also a classification of tissues based on their origin (ontogenetic). According to this classification, primary and secondary tissues are distinguished. From the primary meristem located at the top of the shoot and the tip of the root, as well as from the seed embryo, primary constants tissues (epidermis, collenchyma, sclerenchyma, assimilation tissue, epiblema). Cells of permanent tissues are incapable of further division. From cells of a specialized meristem - procambia - are formed primary conductors tissues (primary xylem, primary phloem).
From the secondary meristem - cambium - are formed secondary tissues: secondary xylem, secondary phloem; from phellogen plug, phelloderm, and lentils are formed, which arise when the stem and root thicken. Secondary tissues are typically found in gymnosperms and dicotyledonous angiosperms. The powerful development of secondary tissues - wood and bast - is characteristic of woody plants.
EDUCATIONAL FABRICS
Educational fabrics Thanks to the constant mitotic division of their cells, they ensure the formation of all plant tissues, i.e. actually shape his body. Any cell in its development goes through three stages: embryonic, growth and differentiation stage (that is, the cell acquires a certain function). As the embryo differentiates, the primary meristem is retained only at the tip of the future shoot (in the growth cone) and at the tip of the root - apical (apical) meristems. The embryo of any plant consists of meristem cells.
Cytological characteristics of meristems. Typical features are most clearly expressed in the apical meristems. These meristems are composed of isodiametric polyhedral cells not separated by intercellular spaces. Their shells are thin, contain little cellulose and are stretchable.
The cavity of each cell is filled with dense cytoplasm with a relatively large nucleus occupying a central position and intensively dividing by mitosis. The hyaloplasm contains many diffusely scattered ribosomes, proplastids, mitochondria and dictyosomes. There are few vacuoles and they are small. Conducting tissues are formed from a meristem that has a prosenchymal shape and large vacuoles - procambium and cambium. Procambium cells are polygonal in cross section, cambium cells are rectangular.
Cells that retain their meristematic properties continue to divide, forming more and more new cells called initials. Some of the daughter cells differentiate, turning into cells of various tissues, they are called derivatives of initials. Initial cells can divide indefinitely many times, and derivatives of initials divide one or more times and develop into permanent tissues.
Based on their origin, primary and secondary meristems are distinguished.
Primary meristems
Primary meristems arise directly from the meristem of the embryo and have the ability to divide. According to their position in the plant, primary meristems can be apical (apical), intercalary (intercalary) and lateral (lateral).
Apical (apical) meristems- such meristems that are located in adult plants at the tops of stems and tips of roots and ensure body growth in length. In the stems, in the growth cone, two meristematic layers are distinguished: the tunica, from which the integumentary tissue and the peripheral part of the primary cortex are formed, and the corpus, from which the inner part of the primary cortex and the central axial cylinder are formed (Fig. 2.3).
Rice. 2.3. Apical meristems of the stem: A- longitudinal section: 1 - growth cone; 2 - leaf primordium; 3 - tubercle of axillary bud;
There are three layers at the root tip:
1) dermatogen, from which the primary integumentary-absorbing tissue - rhizoderm - is formed;
2) periblema, from which the tissues of the primary cortex develop;
3) plerome, forming the tissues of the central axial cylinder.
Lateral (lateral) meristems by origin they can be primary and secondary; on a cross section of the axial organs they look like rings. An example of a primary lateral meristem is the procambium and pericycle. From procambia The cambium and the primary elements of the vascular-fibrous bundles (primary phloem and primary xylem) are formed, while the procambium cells directly differentiate into the cells of the primary conducting tissues.
The lateral meristems are located parallel to the surface of the organ and ensure the growth of the axial organs in thickness.
Intercalary meristems more often they are primary and are preserved in the form of separate areas in zones of active growth in various parts of the plant (for example, at the base of leaf petioles, at the bases of internodes). At the base of internodes in cereals, the activity of this meristem leads to elongation of the internodes, which ensures the growth of the stem in length.
Secondary meristems
Secondary meristems include lateral and wound meristems.
Lateral (lateral) meristems presented cambium And phellogen. They are formed from promeristems (procambium) or permanent tissues by their dedifferentiation. Cambium cells are divided by septa parallel to the surface of the organ (periclinally). Elements of secondary phloem develop from cells deposited outward by the cambium, and elements of secondary xylem develop from cells deposited inward. The cambium, which arose from permanent tissues through dedifferentiation, is called additional In structure and function it does not differ from the cambium, which arose from promeristems. Phellogen is formed from permanent tissues located in the subepidermal layers (under the epidermis). Dividing periclinally, phellogen separates future plug cells (phelleme) outward, and phelloderm cells inward. Thus, phellogen forms secondary integumentary tissue - periderm. The lateral meristems are located parallel to the surface of the organ and ensure the growth of the axial organs in thickness.
Wound meristems are formed when tissues and organs are damaged. Around the damage, living cells dedifferentiate, begin to divide, and thereby transform into a secondary meristem. Their task is to form a dense protective tissue consisting of parenchyma cells - callus. This tissue is whitish or yellowish in color, its cells have large nuclei and fairly thick cell walls. Callus occurs during grafting, ensuring the fusion of the scion with the rootstock, and at the base of the cuttings. It can form adventitious roots and buds, so it is used to obtain isolated tissue cultures.
COVERING TISSUE
Primary integumentary tissue
TO primary integumentary tissues include the epidermis, the epidermal itself, the parastomatal cells, the guard cells of the stomata and the trichomes.
Pectic substances and cellulose included in the cell wall can be subject to mucus formation with the formation slime And gums. They are polymeric carbohydrates related to pectin substances and are characterized by their ability to swell strongly when in contact with water. Gums in a swollen state are sticky and can be pulled out into threads, while mucus is very blurry and cannot be pulled out into threads. Pectic mucilages are found in representatives of the Liliaceae, Cruciferae, Malvaceae, Linden, and Rosaceae families, in contrast to cellulose mucilages, which are much less common (for example, in Orchids).
Stomata
They are highly specialized formations of the epidermis, consisting of two bean-shaped guard cells and a stomatal fissure (a kind of intercellular space between them). They are found mainly in the leaves, but are also found in the stem (Fig. 2.6). 
Rice. 2.6. Stomatal structure: a, b- the skin of a thyme leaf (top view and cross section); V- peel from the stem of Cereus (cactus family); 1 - actual epidermal cells; 2 - guard cells of the stomata; 3 - stomatal fissure; 4 - air cavity; 5 - cells of chlorophyll-bearing parenchyma; A - cuticle; B - cuticular layer - shell with suberin and wax; B - cellulose layer of the wall; G - nucleus with nucleolus; D - chloroplasts
The walls of the guard cells are thickened unevenly: the walls directed towards the gap (abdominal) are significantly thicker compared to the walls directed away from the gap (dorsal). The gap can expand and contract, regulating transpiration and gas exchange. Under the gap there is a large respiratory cavity (intercellular space), surrounded by mesophyll cells of the leaf.
The guard cells are surrounded by parastomatal cells, which together form stomatal complex(Fig. 2.7). The following main types of stomatal complexes are distinguished: 
Rice. 2.7. The main types of stomatal apparatus: 1 - anomocytic (in all higher plants, except horsetails); 2 - diacytic (in ferns and flowering plants); 3 - paracytic (in ferns, horsetails, flowering and oppressive); 4 - anisocytic (only in flowering plants); 5 - tetracytic (mainly in monocots); 6 - ancyclocytic (in ferns, gymnosperms and flowering plants)
1) anomocytic(disorderly) - guard cells do not have clearly defined parastomatal cells; characteristic of all higher plants, excluding conifers;
2) anisocytic(unequal cell) - the guard cells of the stomata are surrounded by three parastomatal cells, one of which is much larger (or smaller) than the others;
3) paracytic(parallel cell) - one parastomatal cell (or more) is located parallel to the guard cells;
4) diacytic(cross-cell) - two parastomatal cells are located perpendicular to the guard cells;
5) tetracite(from Greek tetra- four) - mainly in monocots;
Stomata are located on the underside of the leaf, but in aquatic plants with floating leaves they are found only on the upper side of the leaf. Based on the shape of the leaf epidermal cells and the location of the stomata, a monocotyledonous plant can be distinguished from a dicotyledonous one (Fig. 2.8). The actual epidermal cells of the leaves of dicotyledonous plants are wavy in outline (Fig. 2.9), while in monocotyledonous plants they are elongated, rhombic in shape. 
Rice. 2.8. Location of stomata on the epidermis (view from the surface): A-dicotyledonous plants: 1 - initial letter; 2 - watermelon; b-monocots: 3 - corn; 4 - iris
The types of stomata can be divided according to their level of location relative to the surface of the epidermis as follows.
1.7.1. Stomata located in the same plane as the epidermis. The most common type and is usually not indicated in the description of microscopy of medicinal plant materials, i.e. this paragraph is omitted. Diagnostic signs will be either protruding or submerged stomata.
1.7.2. Protruding stomata - stomata located above the epidermis. Usually, when the microscope microscope is rotated (when the lens is lowered), such stomata are first detected, and only then epidermal cells appear, so it is almost impossible to capture them in a photograph from the surface of a leaf, as well as to depict them in a drawing. In the same plane as the epidermis, such stomata can be seen in transverse sections, but for this, the section must pass through the stomata, which is difficult to obtain given their rare location on the leaf. Such stomata are characteristic, for example, of bearberry leaves.
1.7.3. Submerged stomata - stomata immersed in the epidermis. When observed under a microscope by rotating the microscrew (while lowering the lens), the epidermal cells are first clearly detected, then it becomes possible to more clearly see the contours of the stomata. It is also difficult to display them in photographs and drawings of preparations from the surface. Found in lily of the valley leaves, watch leaves, eucalyptus leaves. Sometimes the recesses in which the stomata are located are lined or covered with hairs and are called stomatal crypts.
1.8. Types of stomatal cells
There are 19 types described in the literature; we have selected only those that are used in the analysis of medicinal plant raw materials**.

Rice. 63. Types of stomatal cells. A - lentiform; B - spherical; B - cap-shaped; G – scaphoid
1.8.1. Lenticular - 2 identical crescent-shaped cells arranged symmetrically. On the frontal plane, the thickening of the shell is almost uniform. The fissure is fusiform (Fig. 63, A). The type of stomatal cells is characteristic of most plants.
1.8.2. Spheroidal - two identical, strongly circularly curved cells are located symmetrically. On the frontal plane, the thickening of the shell is almost uniform. The slot is round (Fig. 63, B).
1.8.3. Cap-shaped - two identical crescent-shaped cells in the polar parts have thickenings in the form of a cap. The fissure is fusiform (Fig. 63, B). Found in foxgloves.
1.8.4. Scaphoid - the internal walls of stomatal cells are thickened. The fissure is fusiform (Fig. 63, D). Observed in centaury grass and watch leaves.
The mechanism of stomata operation is determined by the osmotic properties of cells. When the leaf surface is illuminated by the sun, an active process of photosynthesis occurs in the chloroplasts of the guard cells. Saturation of cells with photosynthetic products and sugars entails the active entry of potassium ions into the cells, as a result of which the concentration of cell sap in the guard cells increases. There is a difference in the concentration of cell sap of the parastomatal and guard cells. Due to the osmotic properties of the cells, water from the parastomatal cells enters the guard cells, which leads to an increase in the volume of the latter and a sharp increase in turgor. Thickening of the “abdominal” walls of the guard cells facing the stomatal fissure ensures uneven stretching of the cell wall; the guard cells acquire a distinct bean-shaped shape, and the stomatal fissure opens. When the intensity of photosynthesis decreases (for example, in the evening), the formation of sugars in guard cells decreases. The influx of potassium ions stops. The concentration of cell sap in guard cells is reduced compared to parastomatal cells. Water leaves the guard cells by osmosis, lowering their turgor; as a result, the stomatal fissure closes at night.
The cells of the epidermis are tightly closed together, thanks to which the epidermis performs a number of functions:
Prevents the penetration of pathogenic organisms into the plant;
Protects internal tissues from mechanical damage;
Regulates gas exchange and transpiration;
Water and salts are released through it;
Can function as suction tissue;
takes part in the synthesis of various substances, the perception of irritations and the movement of leaves.
Trichomes - outgrowths of epidermal cells of different shape, structure and functions: hairs, scales, bristles, etc. They are divided into covering and glandular. glandular trichomes, unlike coverts, they have cells that secrete secretions. Covering hairs forming a woolly, felt or other cover on the plant, they reflect part of the sun's rays and thereby reduce transpiration. Sometimes the hairs are found only where the stomata are located, for example, on the underside of a coltsfoot leaf. In some plants, living hairs increase the total evaporating surface, which helps accelerate transpiration.
Trichome sizes vary significantly. The longest trichomes (up to 5-6 cm) cover cotton seeds. Covering trichomes have the form of simple single or multicellular, branched or stellate hairs. Covering trichomes can remain alive for a long time or quickly die, filling with air.
They differ from trichomes, which arise only with the participation of epidermal cells. emergents, in the formation of which deeper located tissues of the subepidermal layers also participate.
Anatomical and diagnostic features that are of greatest importance and high variability in determining medicinal raw materials. Hairs can be simple or capitate, which in turn can be unicellular or multicellular. Multicellular hairs can be single-row, double-row or branched.
LABORATORY WORK No. 5
WATER EXCHANGE. LEAF AS AN ORGAN OF TRANSPIRATION
Goal of the work: study of the most important functional features of a plant leaf as an organ of transpiration: the structure and number of stomata on the leaf blade, the mechanism of opening and closing stomata, the influence of various substances on the movement of stomata.
TRANSPIRATION
The biological significance of transpiration consists, firstly, in ensuring the constancy of the internal temperature of the leaf. This is achieved by the absorption of heat by water as it evaporates by the leaves. The energy required to transfer a molecule from the liquid phase to the gaseous state without changing temperature is called heat of vaporization. The expenditure of heat on water evaporation is a means of regulating leaf temperature and preventing plants from overheating.
Secondly, transpiration, being the upper end motor, ensures the supply of water and mineral nutrients to the roots. A positive correlation has been established between the intensity of transpiration and the supply of water and ions. If you remove leaves from a plant, the absorption of water by the roots stops. The suction effect of transpiring leaves can be verified by placing a cut branch in a pipette filled with water and lowered into a cup of mercury. After some time, you can observe a rise in the mercury in the pipette, which will indicate a significant suction force of the leaves.
Thus, the rate of water entry into the roots is determined by the intensity of transpiration.
Thirdly, transpiration prevents the occurrence of excess turgor pressure, which could lead to the destruction of plant cells.
Fourthly, the process of transpiration is closely related to plant photosynthesis, which was noted by the works of K. A. Timiryazev. The absorption of CO 2 by plant leaves occurs through stomata, and it depends on the degree of saturation of the leaf tissue with water. The process of assimilation of water and carbon dioxide is a single and inextricable whole.
The rate of transpiration is understood as the amount of water evaporated per unit of time from a unit of leaf surface. Typically this indicator has a dimension of mg/dm2 hour. The amount of water evaporated by plants is quite large and often exceeds the amount of precipitation during the growing season. This excess is compensated by autumn-winter precipitation. For example, one sunflower or corn plant spends 200-250 liters of water over the summer. Wheat plants on an area of 1 hectare evaporate about 2 million liters of water over the summer, corn - more than 3 million, and cabbage - up to 8 million liters. In the process of forming one kilogram of plant mass, 300 liters are consumed. Water.
Stomatal transpiration is regulated by the degree of openness of the stomata. Their structure and distribution depend on the species and environmental characteristics of plants. Stomata are found on all above-ground parts of plants, including reproductive organs and even stamen filaments. The most characteristic stomata are for leaves. More often they are located on the underside of leaves (in mesophytic plants). However, in xerophytes they are also found on the upper side of the leaf.
The average number of stomata per 1 mm 2 area ranges from 100 to 300. The size of stomata does not exceed 20 microns in length and 8-15 microns in width. The total area of open stomata is 1% of the leaf surface.
It has been established that small apical leaves have a greater number of stomata than large lower leaves. The frequency of stomata (their number per unit area) increases when moving from the base of the leaf to its top and from the bottom of the plant to the top. Plants in arid habitats have more of them, but they are smaller in size.
In most mesophytic plants, stomata are located at the same level with the epidermal cells, and in xerophytic forms, stomata are located below the level of the epidermis and are called submerged. In hygrophytes, guard cells are sometimes located above the epidermis. Such stomata are called elevated.
One or another type of stomatal structure is characteristic of certain groups of plants, although within the same family different types of stomata can sometimes be found. Despite the significant area occupied by stomata, the diffusion of water vapor through them accounts for 50-60% of evaporation from the free surface. It has been established that the rate of diffusion through small holes is proportional to their perimeter, not their area. Therefore, partial closure of guard cells has little effect on their perimeter, and the level of diffusion of water vapor through the stomata does not drop very sharply.
Experiment 1. Observation of the movement of stomata under a microscope.
Purpose of experience: determine the dependence of stomata on osmotically active substances.
Materials and equipment: 5% glycerin solution, razor, dissecting needle, microscope, slides and coverslips.
Plants: leaves (Tradescantia, tulip, hydrangea or amaryllis, Kalanchoe).
Gas exchange between leaf intercellular spaces and the external atmosphere is regulated by stomata. Each stoma consists of two guard cells, in which the walls adjacent to the stomatal fissure are greatly thickened, while the outer parts of the shell remain thin. The unequal thickness of the outer and inner walls leads to the fact that when the turgor changes, the guard cells are able to bend or straighten, opening or closing the stomatal fissure.
Progress: sections of the epidermis of a leaf of a selected plant are made, which are placed in a 5% glycerol solution and kept for at least 1 hour. The sections are examined under a microscope, and the degree of opening of the stomatal fissure is determined using an eyepiece micrometer. Make 10 measurements, find the average value and calculate the error of the average. Then the sections are transferred from the glycerol solution to water and the measurements of the stomatal slits are repeated under a microscope. The results are recorded in Table 1.
Table 1
The degree of opening of the stomatal fissure in different environments
|
plant, organ |
Measurement No. |
Degree of opening of the stomatal fissure |
|
|
Glycerol | |||
|
plant leaf | |||
Exercise: draw a conclusion about the effect of glycerol and water on the opening and closing of stomata.
Experiment 2. Determination of the state of stomata and intercellular spaces using the Molisch method
Purpose of experience: will determine the influence of external conditions on the state of stomata and the intensity of transpiration.
Materials and equipment: xylene (in a dropper), ethyl alcohol (in a dropper); benzene (in a dropper), pipettes.
Plant: fresh or withered leaves of plants, leaves of plants that were in the dark.
The intercellular spaces of the leaf are usually filled with air, due to which the leaf appears matte when viewed in the light. If you perform infiltration, i.e. filling the intercellular spaces with any liquid, the corresponding areas of the leaf become transparent.
Determination of the state of stomata by the infiltration method is based on the ability of liquids that wet cell membranes to penetrate by force of capillarity through open stomatal slits into the nearest intercellular spaces, displacing air from them, which can be easily seen by the appearance of transparent spots on the leaf. Different liquids are able to penetrate into stomatal slits that are open to varying degrees: xylene easily penetrates through slightly open stomata, benzene through moderately open stomata, and ethyl alcohol can penetrate only through widely open stomata.
This method, proposed by Molisch, is very simple and quite applicable to work in the field.
Progress. Apply separately small drops of benzene, xylene and ethyl alcohol to the lower surface of the sheet. Keep the sheet in a horizontal position until the drops that can either evaporate or penetrate inside the sheet completely disappear, and examine the sheet in the light.
Examine leaves kept in different conditions (fresh and wilted, illuminated and shaded, etc.). Examine 2-3 sheets each time.
table 2
Influence of external conditions on the degree of stomatal opening
Exercise: Record the results in Table 2, noting the degree of openness of the stomata: wide, medium, weak. Draw a conclusion about the influence of external conditions on stomatal movements.
Experiment 3. Determination of the state of stomata using Molotkovsky prints.
Goal of the work: determination of the work of stomata depending on illumination.
Materials and equipment: colorless nail polish, thin glass rod, tweezers, microscope, eyepiece micrometer, object micrometer.
Plants: indoor plants, the leaves of which are covered with a light-proof cover 2-3 hours before class.
A thin stroke of varnish is applied to the surface of the sheet. After the solvent evaporates, a film is formed on which the epidermis with stomata is imprinted. By examining the resulting prints through a microscope, you can determine the number and size of stomata and measure the width of stomatal slits. This method can be used not only for laboratory, but also for field research (in the latter case, the prints are stored in test tubes with water until determined). To study leaves whose stomata are located in the recesses of the epidermis (for example, in oleander), this method is not applicable, because Such leaves do not produce prints.
Progress. Using a glass rod, apply a drop of varnish solution to the underside of the sheet and quickly spread it in a thin layer. After drying, remove the film with tweezers, place it on a glass slide and examine it at high magnification. Insert an ocular micrometer into the microscope and measure the width and length of the stomatal fissure of at least 10 stomata and calculate the average values.
Determine the division value of the ocular micrometer. To do this, place a micrometer object on the microscope stage, each division of which is equal to 0.01 mm or 10 microns. By turning the eyepiece, align both scales so that their scales are parallel and one overlaps the other. Determination of the division value of an ocular micrometer is carried out according to the vernier principle, i.e. combine one of the scale lines of the ocular and objective micrometer and find the next alignment. Find the matching lines and determine how many divisions of the ocular micrometer A correspond to the divisions of the object micrometer B located between the combined points. The division price of an ocular micrometer is determined by the formula:
Division value = B · 10 µm/A.
By multiplying the length and width of the stomatal openings, expressed in divisions of the ocular micrometer, by the price of one division, find the absolute dimensions of the stomatal slits. Calculate the area of the stomatal fissure with some approximation by multiplying the length by the width.
Examine leaves of different tiers of the same plant, as well as well-lit and shaded ones. Record the results in Table 3.
Table 3
Effect of illumination on the size of stomatal openings
Exercise: draw conclusions about the influence of layering and lighting conditions on the size of stomatal openings.
Although scientists have long known about the evaporation of water by the surface of a leaf, the first to observe stomata was the Italian naturalist Marcello Malpighi, who published this discovery in 1675 in his work Anatome plantarum. However, he did not understand their real function. At the same time, his contemporary Nehemiah Grew developed a hypothesis about the participation of stomata in the ventilation of the internal environment of a plant and compared them with the trachea of insects. Progress in the study came in the 19th century, and then, in 1827, the Swiss botanist Decandolle first used the word “stoma”. The study of stomata at that time was carried out by Hugo von Mohl, who discovered the basic principle of opening stomata, and Simon Schwendener, who classified stomata according to the type of their structure.
Some aspects of the functioning of stomata continue to be intensively studied at the present time; The material is mainly Commelina vulgaris ( Commelina communis), garden bean ( Vicia faba), sweet corn ( Zea mays) .
Structure
The dimensions of the stomata (length) range from 0.01-0.06 mm (the stomata of polyploid plants and leaves growing in the shade are larger. The largest stomata were found in an extinct plant Zosterophyllum, 0.12 mm (120 µm) . The pore consists of a pair of specialized cells called guard cells ( cellulae claudentes), which regulate the degree of openness of the pore; between them there is a stomatal fissure ( porus stomatalis). The walls of the guard cells are thickened unevenly: those directed towards the gap (abdominal) are thicker than the walls directed from the gap (dorsal). The gap can expand and contract, regulating transpiration and gas exchange. When there is little water, the guard cells adhere tightly to each other and the stomatal fissure is closed. When there is a lot of water in the guard cells, it puts pressure on the walls and thinner walls are stretched more, and thicker ones are pulled inward, a gap appears between the guard cells. Under the gap there is a substomatal (air) cavity, surrounded by cells of the leaf pulp, through which gas exchange directly occurs. Air containing carbon dioxide (carbon dioxide) and oxygen enters the leaf tissue through these pores and is further used in the process of photosynthesis and respiration. Excess oxygen produced during photosynthesis by the internal cells of the leaf is released back into the environment through these same pores. Also, during the evaporation process, water vapor is released through the pores. Epidermal cells adjacent to the trailing ones are called accompanying cells (collateral, neighboring, parastomatal). They are involved in the movement of guard cells. The guard and accompanying cells form the stomatal complex (stomatal apparatus). The presence or absence of stomata (the visible parts of stomata are called stomatal lines) are often used in classifying plants.
Types of stomata
The number of accompanying cells and their location relative to the stomatal fissure make it possible to distinguish a number of types of stomata:
- anomocytic - accompanying cells do not differ from other cells of the epidermis, the type is very common for all groups of higher plants, with the exception of conifers;
- diacite - characterized by only two accompanying cells, the common wall of which is at right angles to the guard cells;
- paracytic - accompanying cells are located parallel to the guard cells and stomatal fissure;
- anisocytic - guard cells are surrounded by three accompanying cells, one of which is noticeably larger or smaller than the others, this type is found only in flowering plants;
- tetracytic - four accompanying cells, characteristic of monocots;
- encyclocytic - accompanying cells form a narrow wheel around the guard cells;
- actinocyte - several accompanying cells radiating from the guard cells;
- pericytic - guard cells are surrounded by one secondary accompanying cell, the stomata is not connected to the accompanying cell by an anticlinal cell wall;
- desmocyte - guard cells are surrounded by one accompanying cell, the stomata is connected to it by an anticlinal cell wall;
- polocytic - guard cells are not completely surrounded by one accompanying one: one or two epidermal cells adjoin one of the stomatal poles; the stomata is attached to the distal side of a single accompanying cell, having a U- or horseshoe shape;
- stephanocytic - stomata surrounded by four or more (usually five to seven) poorly differentiated accompanying cells, forming a more or less distinct rosette;
- laterocytic - this type of stomatal apparatus is considered by most botanists as a simple modification of the anomocytic type.
Stomatal location
Dicotyledonous plants, as a rule, have more stomata in the lower part of the leaf than in the upper part. This is explained by the fact that the upper part of a horizontally located leaf, as a rule, is better illuminated, and a smaller number of stomata in it prevents excessive evaporation of water. Leaves with stomata located on the underside are called hypostomatic.
In monocotyledonous plants, the presence of stomata in the upper and lower parts of the leaf is different. Very often the leaves of monocots are arranged vertically, in which case the number of stomata on both parts of the leaf may be the same. Such leaves are called amphistomatic.
Floating leaves do not have stomata on the lower part of the leaf so that they can absorb water through the cuticle. Leaves with stomata located on the upper side are called epistomatic. Underwater leaves have no stomata at all.
The stomata of coniferous plants are usually hidden deep under the endodermis, which makes it possible to greatly reduce water consumption for evaporation in winter, and during drought in summer.
Mosses (with the exception of Anthocerotes) lack true stomata.
Stomata also differ in their level of location relative to the surface of the epidermis. Some of them are located flush with other epidermal cells, others are raised above or buried below the surface. In monocots, whose leaves grow predominantly in length, the stomata form regular parallel rows, while in dicots they are arranged randomly.
Carbon dioxide
Since carbon dioxide is one of the key reagents in the process of photosynthesis, most plants have stomata open during the day. The problem is that when air enters, it mixes with water vapor evaporating from the leaf, and therefore the plant cannot gain carbon dioxide without simultaneously losing some water. Many plants have protection against water evaporation in the form of wax deposits that clog the stomata.
Stomata, which belong to the epidermal tissue system, are of particular importance in the life of a plant. The structure of the stomata is so unique and their significance is so great that they should be considered separately.
The physiological significance of epidermal tissue is dual, largely contradictory. On the one hand, the epidermis is structurally adapted to protect the plant from drying out, which is facilitated by the tight closure of epidermal cells, the formation of a cuticle and relatively long covering hairs. But on the other hand, the epidermis must pass through masses of water vapor and various gases rushing in mutually opposite directions. Gas and steam exchange under some circumstances can be very intense. In a plant organism, this contradiction is successfully resolved with the help of stomata. The stomata consists of two peculiarly modified epidermal cells connected to each other by opposite (along their length) ends and called guard cells. The intercellular space between them is called stomatal fissure.
Guard cells are so called because, through active periodic changes in turgor, they change their shape in such a way that the stomatal fissure either opens or closes. The following two features are of great importance for these stomatal movements. Firstly, guard cells, unlike other cells of the epidermis, contain chloroplasts, in which photosynthesis occurs in the light and sugar is formed. The accumulation of sugar as an osmotically active substance causes a change in the turgor pressure of guard cells compared to other cells of the epidermis. Secondly, the membranes of guard cells thicken unevenly, so a change in turgor pressure causes an uneven change in the volume of these cells, and, consequently, a change in their shape. The change in the shape of the guard cells causes a change in the width of the stomatal fissure. Let's illustrate this with the following example. The figure shows one of the types of stomata of dicotyledonous plants. The outermost part of the stomata consists of membranous projections formed by the cuticle, sometimes insignificant, and sometimes quite significant. They limit a small space from the outer surface, the lower border of which is the stomatal gap itself, called front yard stomata. Behind the stomatal gap, inside, there is another small space, delimited by small internal projections of the side walls of the guard cells, called patio stomata. The patio directly opens into a large intercellular space called air cavity.
In the light, sugar is formed in the guard cells, it draws water from neighboring cells, the turgor of the guard cells increases, and the thin parts of their membrane stretch more than the thick ones. Therefore, the convex projections protruding into the stomatal slit become flat and the stomata opens. If sugar, for example, turns into starch at night, the turgor in the guard cells drops, this causes a weakening of the thin sections of the shell, they protrude towards each other and the stomata closes. In different plants, the mechanism of closing and opening the stomatal gap may be different. For example, in grasses and sedges, guard cells have widened ends and are narrowed in the middle part. The membranes in the middle parts of the cells are thickened, while their expanded ends retain thin cellulose membranes. An increase in turgor causes swelling of the ends of the cells and, as a result, the straight median parts move away from each other. This leads to the opening of the stomata.
Features in the mechanism of operation of the stomatal apparatus are created both by the shape and structure of the guard cells, and by the participation in it of epidermal cells adjacent to the stomata. If the cells immediately adjacent to the stomata differ in appearance from other cells of the epidermis, they are called accompanying cells of stomata.
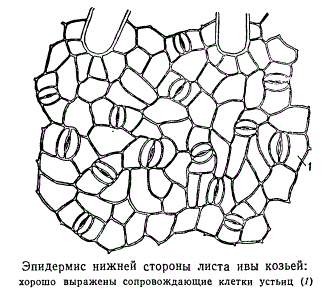
Most often, accompanying and trailing cells have a common origin.
The guard cells of the stomata are either slightly raised above the surface of the epidermis, or, conversely, lowered into more or less deep pits. Depending on the position of the guard cells in relation to the general level of the surface of the epidermis, the very mechanism for adjusting the width of the stomatal fissure changes somewhat. Sometimes the guard cells of the stoma become lignified, and then the regulation of the opening of the stomatal fissure is determined by the activity of neighboring epidermal cells. Expanding and shrinking, i.e. changing their volume, they entrain the guard cells adjacent to them. However, often stomata with lignified guard cells do not close at all. In such cases, regulation of the intensity of gas and vapor exchange is carried out differently (through the so-called beginning drying). In stomata with lignified guard cells, the cuticle often covers with a fairly thick layer not only the entire stomatal fissure, but even extends to the air cavity, lining its bottom.
Most plants have stomata on both sides of the leaf or only on the underside. But there are also plants in which stomata are formed only on the upper side of the leaf (on leaves floating on the surface of the water). As a rule, there are more stomata on leaves than on green stems.
The number of stomata on the leaves of different plants varies greatly. For example, the number of stomata on the underside of an awnless brome leaf is on average 30 per 1 mm 2 , in sunflower growing under the same conditions it is about 250. Some plants have up to 1300 stomata per 1 mm 2 .
In specimens of the same plant species, the density and size of stomata strongly depend on environmental conditions. For example, on the leaves of a sunflower grown in full light, there were an average of 220 stomata per 1 mm 2 of leaf surface, and on a specimen grown next to the first, but with slight shading, there were about 140. On one plant grown in full light, the density stomata increases from the lower leaves to the upper ones.
The number and size of stomata strongly depend not only on the growing conditions of the plant, but also on the internal relationships of life processes in the plant itself. These values (coefficients) are the most sensitive reagents for each combination of factors that determine the growth of a plant. Therefore, determining the density and size of stomata of leaves of plants grown under different conditions gives some idea of the nature of the relationship of each plant with its environment. All methods for determining the size and number of anatomical elements in a particular organ belong to the category of quantitative anatomical methods, which are sometimes used in environmental studies, as well as to characterize varieties of cultivated plants, since each variety of any cultivated plant is characterized by certain limits of size and number of anatomical elements per unit area. Methods of quantitative anatomy can be used with great benefit in both plant growing and ecology.
Along with stomata intended for gas and vapor exchange, there are also stomata through which water is released not in the form of steam, but in a drop-liquid state. Sometimes such stomata are quite similar to ordinary ones, only slightly larger, and their guard cells lack mobility. Quite often, in such a stomata in a fully mature state, guard cells are absent and only a hole remains that leads water out. Stomata that secrete droplets of liquid water are called water, and all formations involved in the release of droplet-liquid water - hydathodes.
The structure of hydathodes is varied. Some hydathodes have parenchyma under the hole that removes water, which is involved in the transfer of water from the water-conducting system and in its release from the organ; in other hydathodes, the water-conducting system directly approaches the outlet. Hydathodes are especially often formed on the first leaves of seedlings of various plants. Thus, in humid and warm weather, the young leaves of cereals, peas and many meadow grasses release water drop by drop. This phenomenon can be observed in the first half of summer in the early morning of every fine day.
The most well-defined hydathodes are located along the edges of the leaves. Often one or more hydathodes are carried by each of the denticles that turn off the edges of the leaves.
For contact of the leaf with the atmosphere there are pores - stomata. Stoma - this is an opening (gap) bounded by two guard cells. Stomata are found in all terrestrial organs of the plant, but most of all in leaves. Each guard cell of the stomata, unlike epidermal cells, has a chloroplast. What happens in them photosynthesis, although with less intensity than in mesophyll cells. Stomata are one of the original devices that have the ability to open and close depending on the saturation of the guard cells with water. Usually stomatal openings are limited two guard cells, the walls of which are unevenly thickened. In dicotyledonous plants, guard cells are bean-shaped, or semi-lunar, in shape, while their internal cell walls adjacent to each other are thicker, and their external ones are thinner. Protoplasts of guard cells are associated in single piece with perforations at the base of the adjacent common walls. When there is little water, the guard cells adhere tightly to each other and the stomatal fissure is closed. When there is a lot of water in the guard cells, it puts pressure on the cell walls, and the thinner walls stretch more, and the thicker ones are pulled inward, and a gap appears between the guard cells.
Recently, it has been proven that the location of stomata is also of great importance. cellulose microfibrils. If usually in leaf cells cellulose fibrils are oriented lengthwise and thickened in this direction, then in the guard cells of stomata the microfibrils are organized radially, which increases resistance to the stretching process.
In cereals the structure of guard cells is somewhat different. They are represented by two elongated cells, at the ends of which the walls are thinner. When saturated with water, the thinner walls at the ends stretch and push the guard cells apart, causing a gap to form.
The number of stomatal openings varies depending on the plant type from 10 to 600 per 1 mm2 of leaf. In many plants (75% of species), including most woody ones, stomata are located on the underside of the leaf. The diameter of the stomatal fissures is only 3-12 microns. Stomata connect the internal spaces of the leaf with the external environment. Water enters the leaf through a network of veins in which vascular elements are located. There are three possible evaporation routes:
- through the stomata - stomatal,
- cuticle - cuticular,
- through lentils - lenticular transpiration.
The distinction between cuticular and stomatal transpiration was first introduced in 1877.
The main types of stomatal apparatus of plant leaves.
- anomocytic (in all higher plants, except horsetails),
- diacytic (in ferns and flowering plants),
- paracytic (in ferns, horsetails, flowering and oppressive),
- anisocytic (only in flowering plants),
- tetracytic (mainly in monocots),
- encyclocytic (in ferns, gymnosperms and flowering plants).
Stomata are highly specialized formations of the epidermis, consisting of two guard cells, between which there is a kind of intercellular space, or stomatal fissure.
The gap can expand and contract, regulating transpiration and gas exchange. Under the gap there is a respiratory, or air, cavity, surrounded by cells of the leaf pulp. The epidermal cells adjacent to the guard cells are called secondary, or parastomatal. They are involved in the movement of guard cells. Guard and subsidiary cells form stomatal apparatus.
The number and distribution of stomata on a leaf or shoot vary depending on the plant species and living conditions. Their number usually ranges from several tens to several hundred per 1 sq. mm of surface.
The mechanism of movement of guard cells is very complex and varies among different species. In most plants, when there is insufficient water supply at night, and sometimes during the day, the turgor in the guard cells decreases and the gap closes, thereby reducing the level of transpiration. With an increase in turgor, the stomata open. It is believed that the main role in these changes belongs to potassium ions. The presence of chloroplasts in guard cells is essential in the regulation of turgor. The primary starch of chloroplasts, turning into sugar, increases the concentration of cell sap. This promotes the influx of water from neighboring cells and the transition of guard cells to an elastic state.
The total area of stomatal openings is only 1-2% of the leaf area. Despite this, transpiration with open stomatal fissures reaches 50-70% of evaporation, equal in area to the open water surface.