What is the resistivity of a material. Electrical resistance of metals. Superconductivity
1. Electrical resistance of metals. The quantum theory of electrical conductivity of metals boils down to the following:
A . In a perfect crystal lattice conduction electrons should not experience resistance during their movement. Resistance occurs when structural defects, that is, the periodicity of the lattice is violated.
b . In real crystals There are two mechanisms for breaking the structure: impurity and thermal. Accordingly, they distinguish impurity resistivity r n And thermal(oscillatory) r T. According to resistance additivity rule metal impedance r equal to their sum, r = r n + r T. (13.1)
V . Impurity resistance r n due to the presence of foreign atoms in the lattice (impurity atoms). If the metal is sufficiently pure and the concentration of impurity atoms is low, then the impurity resistance is practically independent of temperature and becomes noticeable only near absolute zero. Thanks to the impurity, the resistivity of the metal should not go to zero even at T= 0 K.
G . Thermal resistance r T arises due to the scattering of conduction electrons by node density fluctuations crystal lattice, arising from thermal oscillatory motion of nodes. In quantum theory, the thermal vibrational motion of lattice atoms is interpreted as a system of standing sound waves in crystal - phonons. That's why they talk about scattering of conduction electrons by phonons.
In contrast to the classical theory of electrical conductivity of metals Drude - Lorenza, which predicts the dependence of resistance on temperature of the type r~ , quantum theory gives the correct prediction linear dependence r~T. At metal temperatures T³ 50 K r= r 0 aT, which corresponds to the empirical formula r= r 0 (1 + a t). In quantum theory it turns out that when T® 0 total resistivity of the metal r should strive for impurity r n. Figure 90 shows the experimental dependence of the resistivity of pure sodium on temperature.
At T® 0 K r® r n= 4·10 -11 Ohm·m, which is approximately 0.4% of the resistance at T= 273 K. Already at temperatures T³ 20 K dependence r(T) becomes almost linear.
d . Electricity interpreted in quantum theory as electron drift in a periodic crystal field. This drift occurs under the influence of constant electric force her, Where E- tension electric field, creating a current. It turned out that the drift speed of electrons depends on the depth of their position in the conduction band. This dependence is expressed through effective mass m eff electron. Unlike rest mass m e free electron effective mass electron in the conduction band of a metal is a variable value depending on the band width.
Near the bottom of the zone, the effective mass of electrons is positive. The drift direction corresponds to the current density vector. As we rise to the upper boundary of the zone, the effective mass takes on an infinitely large value m eff= ¥ and then becomes negative. Accordingly, the electron drift velocity, having the “correct” direction at the bottom of the zone, gradually passes through zero and takes on negative (“incorrect”) values at the upper boundary of the zone.
Relations obtained in the free electron approximation in theory Drude–Lorenz, turn out to be valid for electrons moving in a periodic lattice field if we replace the rest mass of the electron in them m e to effective m eff.
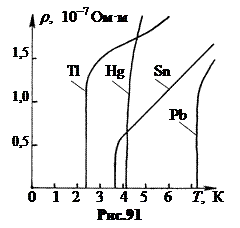 2. Superconductivity. In 1911 Kamerling – Onnes, measuring the resistance of mercury in the low temperature region, discovered that when T= 4.2 K, the resistance of mercury practically dropped to zero. This phenomenon came to be called superconductivity. Figure 91 shows experimental curves of the dependence of the resistivity of some pure metals on temperature near absolute zero. Obviously, the phenomenon cannot be reduced to a normal drop in the resistivity of a defect-free crystal, when r n= 0, and r T. The transition to the superconducting state does not occur smoothly, but abruptly at a certain temperature T cr which is called critical transition temperature. About 30 superconducting chemical elements and over 500 superconducting materials are now known.
2. Superconductivity. In 1911 Kamerling – Onnes, measuring the resistance of mercury in the low temperature region, discovered that when T= 4.2 K, the resistance of mercury practically dropped to zero. This phenomenon came to be called superconductivity. Figure 91 shows experimental curves of the dependence of the resistivity of some pure metals on temperature near absolute zero. Obviously, the phenomenon cannot be reduced to a normal drop in the resistivity of a defect-free crystal, when r n= 0, and r T. The transition to the superconducting state does not occur smoothly, but abruptly at a certain temperature T cr which is called critical transition temperature. About 30 superconducting chemical elements and over 500 superconducting materials are now known.
 3. Effects of superconductivity.
3. Effects of superconductivity.
A . Electricity , excited in a superconducting ring, can circulate in it for years.
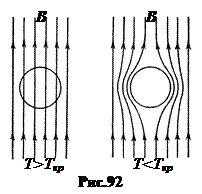
b . Effect Meisner. In 1933 Walter Meissner And R. Oxenfeld discovered that a substance placed in a magnetic field (Fig. 92 on the left), when transitioning to a superconducting state, does not freeze the magnetic field located in it, as should have been the case when the substance simply transitioned to a state with zero resistance, but pushes it out of its volume (Fig. 92 on the right). This is inherent ideal diamagnetic materials with zero magnetic permeability m= 0.
Since the magnetic field does not penetrate the superconductor, it follows that electric current can only flow along the surface of the superconductor. After all, if a current could flow in the thickness of a superconductor, then there would be a magnetic field around it in the thickness of the superconductor. Indeed, experience shows that electric current flows in a superconductor in a surface layer of thickness l= 10 ¸ 100 nm. The magnetic field also penetrates into the superconductor to this depth, decreasing with distance x from the surface according to the exponential law
B = B 0 exp(- xçl). (13.2)
A substance in a superconducting state acquires two fundamental properties unrelated to each other: ideal conductivity and ideal diamagnetism.
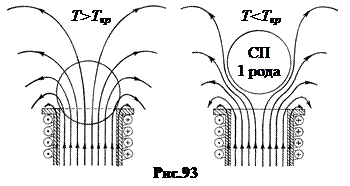 Effect Meisner allows you to stably suspend superconducting bodies in a magnetic field (Fig. 93). When the ball transitions to a type 1 superconducting state, the magnetic field is displaced from it. As a result, a current is induced in the surface layer of the ball in such a direction that the ball is pushed out of the field.
Effect Meisner allows you to stably suspend superconducting bodies in a magnetic field (Fig. 93). When the ball transitions to a type 1 superconducting state, the magnetic field is displaced from it. As a result, a current is induced in the surface layer of the ball in such a direction that the ball is pushed out of the field.
 V . Critical effect magnetic field
.
It consists in the fact that when the magnetic field in which the superconductor is located reaches a certain limiting value of induction In kr»10 -2 ¸ 10 1 T, superconductivity disappears.
V . Critical effect magnetic field
.
It consists in the fact that when the magnetic field in which the superconductor is located reaches a certain limiting value of induction In kr»10 -2 ¸ 10 1 T, superconductivity disappears.
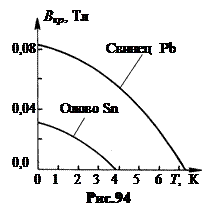
Figure 94 shows the dependence In kr on temperature for lead (upper curve) and for tin (lower curve). At critical temperature T = T cr the critical field is zero, In kr= 0, and with decreasing temperature In kr increases.
If we increase the current flowing through a superconductor, then at some critical value I cr the superconducting state is destroyed. Since the magnetic field IN proportional to current I, then the dependence I cr on temperature is similar to the dependence In kr(T). The critical magnetic field effect complicates the technique of producing superstrong magnetic fields using superconducting circuits. The calculation of the critical current must take into account that the current flows in the surface layer. For example, for a conductor with a diameter of 1 mm at l = 35 nm cross-section of the surface layer through which current flows is about 10 -4 mm 2. This is about 0.01% of the total conductor cross-section.
 d. Effect Josephson
. In 1962 Brian Josephson theoretically predicted two effects, the essence of which is as follows.
d. Effect Josephson
. In 1962 Brian Josephson theoretically predicted two effects, the essence of which is as follows.
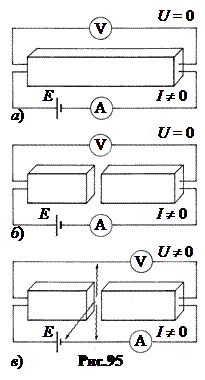
Let's connect an ammeter to the superconductor (in Fig. 95-a it is shown in the form of a bar) A with a direct current source, the emf of which E, and a voltmeter V. There is a direct current in the circuit, which is recorded by an ammeter. Since the resistance of the superconductor is zero, the voltmeter shows zero.
Let's cut the superconductor into two parts and move them apart so that a gap of thickness appears between them d» 1 nm. As predicted Josephson, when such a superconductor is connected to a circuit, one of the following two effects can be observed.
Stationary Josephson effect. A direct current still flows through the superconductor. It turns out that current can flow without resistance not only through a superconductor, but also through a gap in it, if it is narrow enough (Fig. 95-b).
Non-stationary Josephson effect. At the ends of a superconductor with a gap, constant potential difference. In this case, it is emitted from the slit high frequency electromagnetic wave(Fig.95-c). Not only direct, but also high-frequency alternating current flows through the superconductor.
Currently the effects Josephson not only confirmed experimentally, but also used in microelectronics.
4. The theory of superconductivity built in 1957 John Bardeen, Leon Cooper And John Schrieffer. Based on the first letters of their last names, they named her BKSH – theory. The BCS theory is based on the idea that conductivity electrons in a metal can act gravity, arising due to their polarization of the crystal lattice.
An electron moving in a lattice attracts positively charged ions, bringing them somewhat closer together, and thereby creates an excess positive charge of a polarized lattice along its path, to which other electrons can be attracted. This is equivalent to the emergence of an attractive force between electrons, only acting not directly, but through a polarized lattice.
 It can be assumed that superconductivity should be expected primarily in those metals in which there is a strong interaction of the electron gas with the lattice, leading under normal conditions to high resistivity. And indeed, of the pure metals, the best superconductors turned out to be those with the highest resistance - lead Pb, niobium Nb, tin Sn, mercury Hg. At the same time, such low-resistivity metals as copper Cu and silver Ag, in which the electron gas has high mobility, do not exhibit superconductivity.
It can be assumed that superconductivity should be expected primarily in those metals in which there is a strong interaction of the electron gas with the lattice, leading under normal conditions to high resistivity. And indeed, of the pure metals, the best superconductors turned out to be those with the highest resistance - lead Pb, niobium Nb, tin Sn, mercury Hg. At the same time, such low-resistivity metals as copper Cu and silver Ag, in which the electron gas has high mobility, do not exhibit superconductivity.
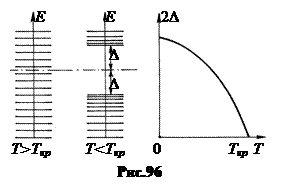
As shown Leon Cooper, at T< T cr, the top ones electrons located at the Fermi level can pair. Wherein their total energy turns out to be less than the sum of the energies of individual electrons. The released energy must be removed from the crystal by cooling. Energy loss Cooper pairs leads to a decrease in the upper level occupied by electrons. As a result, a band gap of 2D width appears between the levels of Cooper pairs and the nearest free levels (Fig. 96 on the left). This resulting energy gap does not allow Cooper pairs of electrons to accept low energy. They can only accept energy of at least 2D, which will allow electrons to jump across this gap. Therefore, when T< T cr Cooper pairs turn out to be very stable.
At T< T cr Not all electrons are paired. At each temperature, a certain equilibrium relationship is established between the concentrations of normal and paired electrons. It turns out that the width is 2 D The energy gap in a superconductor depends on the number of unpaired electrons. Their concentration decreases with decreasing temperature and the width of the gap increases accordingly (Fig. 96 on the right).
The electrons forming Cooper pairs have opposite spins. Therefore, the spin of the pair is zero, and it represents boson. Bosons can accumulate in the ground energy state, from which they are difficult to transfer to an excited state. Therefore, Cooper pairs can remain in a state of coordinated motion indefinitely. This coordinated movement of pairs is the superconductivity current.
The distance between the pair's electrons is large. It is approximately 1000 nm, which is about 5000 atomic diameters. Approximately 1000 pairs overlap, occupying the total volume.
5. Explanation of the BCS - the theory of the critical current effect. For known superconductors, the energy gap is on average 2D = 3 meV » 5·10 -22 J. To destroy a Cooper pair, one of the electrons of the pair must reduce the energy of its motion by at least 2D.
Let us assume that the electron gives up this energy in a head-on collision with a lattice site so that after the collision it rebounds with the same drift speed v d in the opposite direction. Electron energy before collision E k1 = m e(v f + v d) 2 ç 2, energy after collision E k2 = m e(v f - v d) 2 ç 2. Here v f– thermal speed of electrons at the Fermi level (»10 6 m ç With), v d– electron drift speed in electric field, it does not exceed 1 m ç With.
The loss of electron kinetic energy must be at least equal to 2D. So D E k = = 2 m e v f v d= 2D. (13.3)
Hence, the minimum drift speed v d, necessary for the destruction of a Cooper pair, is v d=D çm e v f. (13.4)
The electron conduction current density is j = env d, (13.5)
Where n– concentration of conduction electrons in the metal. Substituting the critical drift speed from (13.4), we obtain the critical current density j cr .
j cr = env d= en D çm e v f. (13.6)
In typical superconductors n= 3·10 28 m -3, v f= 10 6 m ç s, 2D = 3 meV. Let's substitute.
j cr = ![]() =10 12 . This corresponds to a current of 10 6 A through a conductor with a cross section of 1 mm 2. But in a real superconductor, the current flows only in a thin near-surface layer about 35 nm thick, which corresponds to the cross section S= 10 -4 mm 2. Therefore, the critical current in a superconductor about 1 mm thick is only i cr = j cr S= 10 6 A ç
mm 2 ·10 - 4 mm 2 = 100 A. This is quite consistent with the experiment.
=10 12 . This corresponds to a current of 10 6 A through a conductor with a cross section of 1 mm 2. But in a real superconductor, the current flows only in a thin near-surface layer about 35 nm thick, which corresponds to the cross section S= 10 -4 mm 2. Therefore, the critical current in a superconductor about 1 mm thick is only i cr = j cr S= 10 6 A ç
mm 2 ·10 - 4 mm 2 = 100 A. This is quite consistent with the experiment.
6. Explanation of the critical magnetic field by the BCS theory. When placing a superconductor in a magnetic field IN a continuous current is induced in the surface layer of the superconductor. This undamped current has such a magnitude and direction that its magnetic field inside the superconductor completely compensates for the external field IN . As the field increases IN the compensating current density in the superconductor increases. If the external field IN will be so large that the density of the induction current induced by it will reach a critical value, superconductivity will be destroyed.
All of the above applies to superconductors 1-cities, in which the electric current exists only in the near-surface layer. Somewhat later they were discovered and studied superconductors 2-cities. In them, arising in an external magnetic field IN superconducting currents flow not only along the surface, but also penetrate into the thickness of the conductor. Type 1 superconductors have a critical magnetic field IN cr does not exceed 0.1 T, and for type 2 superconductors it reaches the value IN cr » 20 Tl.
7. Effects Josephson explained by BCS theory as a result of tunneling of Cooper pairs through a narrow gap between superconductors. According to theory, frequency n alternating superconducting current is determined by the expression: n= . (13.7)
When there is tension on the gap U= 1 mV frequency n= 485 GHz, which corresponds to the wavelength of EM radiation l = сçn= 0.6 mm.
8. Superconductor reactance. At any temperature T< T A cr superconductor almost always contains both superconducting electrons with a concentration n c, and normal ( n n) electrons. If you place a superconductor in a high-frequency field, then in this alternating electric field not only Cooper pairs, but also normal electrons are accelerated. Therefore, the current has both a superconducting and a normal component.
Both electrons have mass, and due to their inertia, the current lags in phase with the RF field strength. Cooper pairs move in the conductor as if without friction. According to classical mechanics, the speed of particles in this case lags in phase from the periodic force acting on them by pç 2. Therefore, the superconducting component of the high-frequency current lags behind the field strength by pç 2. This means that Cooper pairs create purely reactance.
Normal electrons move as if with friction. Therefore, they create both reactive and active resistance.
The most significant contribution to residual resistance comes from scattering by impurities, which are always present in a real conductor either as contamination or as an alloying (i.e. intentionally introduced) element. Any impurity additive leads to an increase in g, even if it has increased conductivity compared to the base metal. Thus, the introduction into a copper conductor is 0.01 at. the proportion of silver impurity causes an increase in the resistivity of copper by 0.002 μΩ m. It has been experimentally established that at a low impurity content, the resistivity increases in proportion to the concentration of impurity atoms. Different impurities have different effects on the residual resistance of conductors. In addition to impurities, some contribution to the residual resistance is made by intrinsic structural defects - vacancies, interstitial atoms, dislocations, grain boundaries.
The reasons for the scattering of electron waves in a metal are not only thermal vibrations of lattice nodes, but also static structural defects, which also disrupt the regularity of the crystal lattice. Scattering from static structural defects does not depend on temperature. Therefore, as the temperature approaches absolute zero, the resistance of real metals tends to a certain constant value, called residual resistance.
The resistivity of a substance depends on temperature. As a rule, the resistance of metals increases with temperature. This should not be surprising: as temperature increases, atoms move faster, their arrangement becomes less ordered, and we can expect them to interfere more with the flow of electrons. In narrow temperature ranges, the resistivity of the metal increases almost linearly with temperature:
At very low temperatures The resistivity of some metals, as well as alloys and compounds, drops to zero within the accuracy of modern measurements. This property is called superconductivity; it was first observed by the Dutch physicist Geiko Kamer-Ling-Onnes (1853-1926) in 1911 when mercury was cooled below 4.2 K. At this temperature, the electrical resistance of mercury suddenly dropped to zero.
It is worth noting that among good conductors, which are metals, precious metals are the most preferred, while silver is considered the best conductor, because it has the lowest resistivity. This explains the use precious metals especially when soldering important elements in electrical engineering. From the resistivity values of substances, one can judge their practical application- substances with high resistivity are suitable for manufacturing insulating materials, and with a small amount - for conductors.
To obtain the dependence of the current in the circuit on the resistance, Ohm had to conduct a huge number of experiments in which it was necessary to change the resistance of the conductor. In this regard, he was faced with the problem of studying the resistance of a conductor depending on its individual parameters. First of all, Georg Ohm drew attention to the dependence of the resistance of a conductor on its length, which was already briefly discussed in previous lessons. He concluded that as the length of the conductor increases, its resistance also increases in direct proportion. In addition, it was found that the resistance is also affected by the cross-section of the conductor, i.e. the area of the figure, which is obtained from a cross-section. Moreover, the larger the cross-sectional area, the lower the resistance. From this we can conclude that the thicker the wire, the lower its resistance. All these facts were obtained experimentally.
If you pay attention to this formula, you can conclude that it expresses the resistivity of the conductor, i.e., by determining the current and voltage on the conductor and measuring its length with cross-sectional area, you can use Ohm’s law and the specified formula to calculate resistivity. Then, its value can be compared with the data in the table and determine what substance the conductor is made of.
The number of charge carriers in a metal conductor remains virtually unchanged with increasing temperature. However, due to increased vibrations of crystal lattice nodes with increasing temperature, more and more obstacles appear in the path of the directional movement of free electrons under the influence of an electric field, i.e., does the mean free path of an electron decrease? , The mobility of electrons decreases and, as a result, the conductivity of metals decreases and the resistivity increases (Figure 2.1)
As already indicated, impurities and disturbances in the correct structure of metals increase their resistivity. Significant growth? observed during an alloy of two metals if they form a solid solution with each other, i.e., upon solidification, they crystallize together, and the atoms of one metal enter the crystal lattice of the other.
This coefficient is interesting not only when considering the work of various conjugate materials in a particular structure (the possibility of cracking or breaking the vacuum tight connection with glass, ceramics when temperature changes). It is also necessary to calculate the temperature coefficient of wire resistance
When two different metal conductors collide, a contact potential difference occurs between them. The reason for the appearance of this potential difference is the difference in the work function of electrons from different metals, and also in the fact that the electron concentration, and, consequently, the pressure of the electron gas in different metals and alloys may be different. From the electronic theory of metals it follows that the contact potential difference between metals A and B is equal to
These difficulties were overcome by adopting the position of quantum mechanics. In contrast to classical electronic theory, quantum mechanics believes that electron gas in metals at ordinary temperatures is in a state of vibrancy. In this state, the energy of the electron gas is almost independent of temperature, i.e. thermal motion almost does not change the energy of electrons. Therefore, heat energy is spent on heating the electron gas, and it turns out when measuring the heat capacity of metals. Electron gas reaches a state similar to ordinary gases at temperatures of the order of thousands of kelvins. By representing a metal as a system in which positive ions are held together by freely mobile electrons, it is easy to understand the nature of all the basic properties of metals: plasticity, malleability, good thermal conductivity and high electrical conductivity.
Sodium metal is a fairly promising conductor material. Sodium can be obtained by electrolysis of molten sodium chloride NaCl in virtually unlimited quantities. From a comparison of the properties of sodium with the properties of other conductive metals, it is clear that the resistivity of sodium is approximately 2.8 times greater? copper and 1.7 times more? aluminum, but due to the extremely low density of sodium (its density is almost 9 times less than the density of copper), a sodium wire with a given conductivity per unit length should be significantly lighter than a wire made of another metal. However, sodium is extremely active chemically (it oxidizes intensely in air and reacts violently with water), so the sodium wire must be protected by a sealing sheath. The sheath must provide the wire with the necessary mechanical strength, since sodium is very soft and has a low tensile strength during deformation.
Iron (steel) as the cheapest and available metal, which also has high mechanical strength, is of great interest for use as a conductor material. However, even pure iron has a significantly higher resistivity compared to copper and aluminum; ? steel, that is, iron mixed with carbon and other elements, is even higher. Ordinary steel has low corrosion resistance: even at normal temperatures, especially in conditions high humidity, it rusts quickly; As the temperature rises, the corrosion rate increases sharply. Therefore, the surface of steel wires must be protected with a layer of more resistant material. Zinc coatings are usually used for this purpose.
This is the electrical resistance per unit length of a conductor per unit cross-sectional area [Ohm m], exerted by the movement of charge carriers in the conductor, as well as semiconductor ions in solutions, under the influence of a potential electric field. Electrical resistivity DC on the one hand, it is a derived concept from the electrical resistance of a conductor, and on the other -Basic concept electrical engineering materials science, since it determines the properties of the conductor material regardless of its length and shape in general.
The ability of metals to change their resistance with changes in temperature is used to construct resistance thermometers. This thermometer is a platinum wire wound on a mica frame. By placing a thermometer, for example, in a furnace and measuring the resistance of the platinum wire before and after heating, the temperature in the furnace can be determined.
In metals, the Fermi level lies in the conduction band, which is only partially filled. Electrons located in the conduction zone, having received an arbitrarily small energy addition (for example, due to thermal motion or an electric field), can move to a higher (free) energy level in the same zone, that is, become free electrons and participate in conduction. As the temperature increases, the resistance will increase, since the scattering of conduction electrons by thermal vibrations of the lattice increases, and the average free path of the electron decreases.
(everywhere below, resistance is understood as active (resistive) resistance, in which dissipation (dissipation) of electrical energy occurs and its irreversible transition into other types of energy, for example, thermal)
At absolute zero in an ideally perfect crystal the atoms are arranged strictly periodically and electromagnetic waves pass freely through the crystal lattice without experiencing resistance. In real conditions, metal conductors have a distorted lattice and are used at temperatures other than absolute zero.
As the temperature increases, metal atoms vibrate around the lattice nodes, causing electron waves to scatter, leading to an increase in electrical resistance. This increase can be expressed by the dependence
For very small deformations, a decrease in resistance is sometimes observed, which should be attributed to side effects: metal compaction, destruction of insulating intergranular films, etc.
The occurrence of ordering in solid solutions is the result of an increase in the chemical interaction of the components, as a result of which electrons are bound more strongly than in an unregulated solid solution. Increasing the chemical interaction of the components reduces the number of conduction electrons and increases the residual electrical resistance. However, when composed, the electric field of the ionic core of the lattice becomes more symmetrical, and this naturally leads to a decrease in the residual resistance. The latter circumstance turns out to prevail, and during composition the electrical resistance decreases.
2. Conductor materials
2.1. General information about conductors
As guides electric current Both solids and liquids can be used, and under appropriate conditions (in a state of ionization) also gases.
From metal conductor materials can be distinguished high conductivity metals having a resistivity at normal temperature of no more than 0.05 μOhm m, and high resistance alloys with a resistivity of at least 0.3 μOhm m.
Of particular interest are materials with extremely low resistivity at very low temperatures. superconductors And cryoconductors .
Liquid conductors include molten metals and electrolytes. For most metals, the melting point is high; only mercury, which has a melting point of minus 39°C, can be used as a liquid metal conductor at normal temperature. Other metals are liquid conductors only at elevated temperatures.
The mechanism for the passage of current in metals - both in solid and liquid states - is determined by the movement of free electrons under the influence of an electric field; That's why metals are called conductors with electronic conductivity or conductors of the first kind . Guides of the second kind, or electrolytes, are solutions, in particular aqueous, acids, alkalis and salts. The passage of current through these substances is associated with the transfer of ions along with electrical charges in accordance with Faraday's laws, as a result of which the composition of the electrolyte gradually changes, and electrolysis products are released on the electrodes. Ionic crystals in the molten state are also conductors of the second kind. An example is salt quenching baths with electric heating.
All gases and vapors, including metal vapors, are not conductors at low electric field strengths. However, if the field strength exceeds a certain critical value that ensures the onset of impact and photoionization, then the gas can become a conductor with electronic and ionic conductivity. A highly ionized gas with an equal number of electrons to the number of positively charged ions per unit volume represents a special conducting medium called plasma .
2.2. Electrical conductivity of metals
The classical electronic theory of metals represents a conductor as a system consisting of nodes of an ionic crystal lattice, inside which there is an electron gas of free electrons. From one to two electrons pass from each atom into a free state. The concepts and laws of statistics of ordinary gases were applied to the electron gas. Considering the thermal and directional motion of electrons under the influence of an electric field, we obtained the expression of Ohm's law. When electrons collide with nodes of a crystal lattice, the energy accumulated during the acceleration of electrons in an electric field is transferred to the metal base of the conductor, as a result of which it heats up. Consideration of this process led to the derivation of the Joule-Lenz law. Thus, the electronic theory of metals made it possible to theoretically describe and explain the basic laws of electrical conductivity and losses previously found experimentally electrical energy in metals. It also turned out to be possible to explain the relationship between the electrical and thermal conductivity of metals.
However, contradictions between some theoretical conclusions and experimental data also appeared. They consisted of a discrepancy between the temperature dependence curves of resistivity and a discrepancy between the theoretically obtained values of the heat capacity of metals and the experimental data.
These difficulties were overcome by adopting the position of quantum mechanics. In contrast to classical electron theory, quantum mechanics believes that the electron gas in metals at ordinary temperatures is in a state of degeneracy. In this state, the energy of the electron gas is almost independent of temperature, i.e. thermal motion almost does not change the energy of electrons. Therefore, heat is not spent on heating the electron gas, which is revealed when measuring the heat capacity of metals. Electron gas reaches a state similar to ordinary gases at temperatures of the order of thousands of Kelvin. By imagining a metal as a system in which positive ions are held together by freely moving electrons, it is easy to understand the nature of all the basic properties of metals: plasticity, malleability, good thermal conductivity and high electrical conductivity.
2.3. Properties of conductors
TO the most important parameters characterizing the properties of conductor materials include:
- specific conductivity g or its inverse value – resistivity r,
- temperature coefficient of resistivity TKr or a r,
- thermal conductivity g t,
- contact potential difference and thermo-emf,
- work function of electrons leaving the metal,
- tensile strength s r and elongation at break Dl/l.
2.3.1. Conductivity and resistivity of conductors
The relationship between current density J, A/m 2, and electric field strength E, V/m, in a conductor is given by the well-known formula:
Here g, S/m is a parameter of the conductor material, called its conductivity ; in accordance with Ohm's law, g does not depend on the electric field strength when the latter changes within a very wide range. The value r=1/g, the reciprocal of specific conductivity and called resistivity , for a conductor with a resistance R of length l with a constant cross-section S is calculated by the formula
ρ = R·S/l. (2.2)
The SI unit for resistivity is ohm m. The range of resistivity values ρ of metal conductors at normal temperature is quite narrow: from 0.016 for silver to approximately 10 μΩ m for iron-chromium-aluminum alloys, i.e. it occupies only three orders of magnitude. The value of specific conductivity γ mainly depends on the mean free path of electrons in a given conductor, which, in turn, is determined by the structure of the conductor material. All pure metals with the most regular crystal lattice are characterized by the lowest resistivity values; impurities, distorting the lattice, lead to an increase in ρ. And from the point of view of wave theory, scattering of electron waves occurs on crystal lattice defects, which are comparable to a distance of the order of a quarter of the electron wavelength. Smaller faults do not cause noticeable wave scattering.
2.3.2. Temperature coefficient of resistivity of metals
The number of charge carriers in a metal conductor remains practically unchanged with increasing temperature. However, due to vibrations of the crystal lattice nodes, with increasing temperature, more and more obstacles appear in the path of the movement of free electrons directed under the influence of the electric field, i.e. the mean free path of an electron decreases, the mobility of electrons decreases and, as a result, the conductivity of metals decreases and the resistivity increases. In other words, the temperature coefficient of resistivity of metals is positive.
2.3.3. Change in resistivity of metals during melting
When transitioning from a solid to a liquid state, most metals experience an increase in resistivity, as can be seen from Fig. 2.1; however, some metals increase ρ when melted.
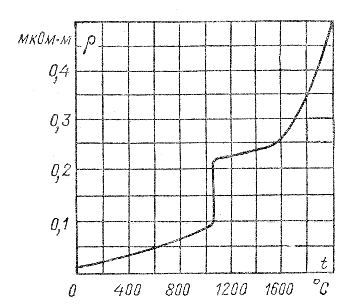
The jump corresponds to the copper melting temperature of 1083°C
The resistivity increases during melting for those metals that increase their volume during melting, i.e. reduce density; for metals with the opposite nature of volume change during melting (similar to the ice-water phase transition), ρ decreases.
2.3.4. Change in resistivity of metals during deformation
The change in resistivity during tension or compression can be approximately estimated by the formula
ρ = ρ 0 (1± σ ·s) , (2.3)
where ρ is the resistivity of the metal under mechanical stress σ, ρ 0 is the resistivity of the metal not subject to mechanical stress, s is the mechanical stress coefficient characterizing the given metal; The plus sign in the formula corresponds to stretching, the minus sign to compression.
The change in ρ during elastic deformation is explained by a change in the vibration amplitude of the nodes of the metal crystal lattice. When stretched, these amplitudes increase, and when compressed, they decrease. An increase in the vibration amplitude of crystal lattice sites leads to a decrease in the mobility of charge carriers and, as a consequence, to an increase in ρ. Plastic deformation, as a rule, increases the resistivity of metals due to distortion of the crystal lattice. During recrystallization by annealing, the resistivity can be reduced again to its original value.
2.3.5. Alloy resistivity
A significant increase in ρ is observed when two metals are alloyed if they form with each other solid solution , i.e. During solidification, they create joint crystallization, and the atoms of one metal enter the crystal lattice of another. ρ has a maximum corresponding to a certain ratio between the content of components in the alloy. Thus, N.S. Kurnakov discovered that in those cases when, at a certain ratio between the components, they form distinct chemical compounds with each other ( intermetallic compounds ), kinks are observed on the ρ curves as a function of composition (Fig. 2.2).
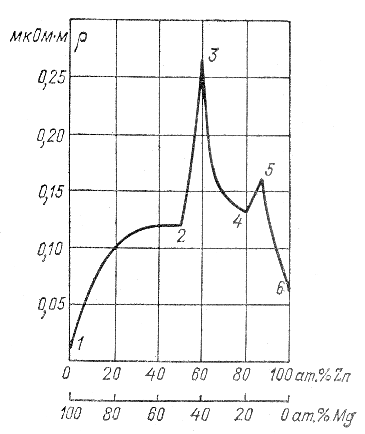
Rice. 2.2. Dependence of resistivity of zinc – magnesium alloys on composition.
Point 1 corresponds to pure Mg, 2 to compound
MgZn, 3 - Mg 2 Zn 3, ., 4 – MgZn 4 5 – MgZn 6, 6 – pure Zn.
Research by A.F. Ioffe showed that many intermetallic compounds are not substances with metallic conductivity, but electronic semiconductors.
If an alloy of two metals creates separate crystallization, and the structure of the solidified alloy is a mixture of crystals of each component (i.e., there is no distortion of the crystal lattice of each component), then the specific conductivity γ of the alloy changes with a change in composition approximately linearly, i.e. . determined by the arithmetic mixing rule (Fig. 2.3).
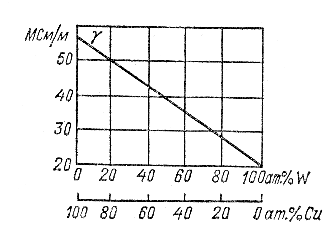
Fig.2.3. Dependence of the conductivity of copper-tungsten alloys on composition (in percent by weight)
2.3.6. Thermal conductivity of metals
The same free electrons that determine the electrical conductivity of metals, and the number of which per unit volume is very large, are mainly responsible for the transfer of heat through a metal. Therefore, as a rule, the thermal conductivity γ t of metals is much greater than the thermal conductivity of dielectrics. It is obvious that, other things being equal, the greater the specific electrical conductivity γ of a metal, the greater its thermal conductivity should be. It is also easy to see that with increasing temperature, when the mobility of electrons in the metal and, accordingly, its specific conductivity decrease, the ratio γ t / γ δ should increase.
The purity and nature of the mechanical processing of the metal can significantly affect its thermal conductivity, especially at low temperatures.
2.3.7. Thermoelectromotive force
When two metal conductors come into contact, a contact potential difference . The reason for its appearance is the difference in the work function of electrons from different metals, as well as the fact that the electron concentration, and therefore the electron gas pressure, may not be the same for different metals and alloys. From the electronic theory of metals it follows that the contact potential difference between metals A and B is equal to:
![]() (2.4)
(2.4)
where U A and U B are the potentials of contacting metals; n A and n B are the electron concentrations in metals A and B.
If the temperatures of the “junctions” are the same, then the sum of the potential differences is equal to zero. The situation is different when one metal has a temperature T 1, and the other - T 2.
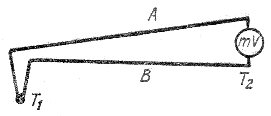
In this case, a thermo-emf arises between the “junctions” equal to
which can be written in the form
Where c is the thermo-emf coefficient constant for a given pair of conductors, i.e. thermo-emf must be proportional to the temperature difference between the metals.
A wire made up of two wires made of different metals or alloys insulated from each other ( thermocouple ), can be used to measure temperatures.
2.3.8. Mechanical properties of conductors
They are characterized by tensile strength σ р and relative elongation at break Δl/l, as well as brittleness, hardness and similar properties. The mechanical properties of metal conductors largely depend on mechanical and thermal treatment, on the presence of alloying impurities, etc. The effect of annealing leads to a significant decrease in σ р and an increase in Δl/l. Parameters of conductor materials such as boiling and melting points, specific heat capacity, etc. do not require special explanation.
2.4. High conductivity materials
The most widely used high conductivity materials include copper and aluminum.
2.4.1. Copper
The advantages of copper, which ensure its widespread use as a conductor material, are as follows:
- low resistivity;
- sufficiently high mechanical strength;
- corrosion resistance is satisfactory in most applications;
- good workability: copper is rolled into sheets, strips and drawn into wire, the thickness of which can be increased to thousandths of a millimeter;
- relative ease of soldering and welding.
Copper is most often obtained by processing sulfide ores. After a series of ore smelting and roasting with intense blasting, copper intended for electrical purposes must undergo a process of electrolytic purification.
Copper grades M1 and M0 are most often used as conductor material. M1 grade copper contains 99.9% Cu, and in the total amount of impurities (0.1%) oxygen should be no more than 0.08%. The presence of oxygen in copper worsens its mechanical properties. The best mechanical properties are found in M0 grade copper, which contains no more than 0.05% impurities, including no more than 0.02% oxygen.
Copper is a relatively expensive and scarce material, so it is increasingly being replaced by other metals, especially aluminum.
In some cases, alloys of copper with tin, silicon, phosphorus, beryllium, chromium, magnesium, and cadmium are used. Such alloys, called bronzes, with the correct composition, have significantly higher mechanical properties than pure copper.
2.4.2. Aluminum
Aluminum is the second most important conductor material after copper. This is the most important representative of the so-called light metals: the density of cast aluminum is about 2.6, and rolled aluminum is 2.7 Mg/m3. Thus, aluminum is approximately 3.5 times lighter than copper. The temperature coefficient of expansion, specific heat capacity and heat of fusion of aluminum are greater than those of copper. Due to the high specific heat capacity and heat of fusion, heating aluminum to the melting point and transferring it to a molten state requires big expense heat than to heat and melt the same amount of copper, although the melting point of aluminum is lower than copper.
Aluminum has lower properties compared to copper - both mechanical and electrical. With the same cross-section and length, the electrical resistance aluminum wire 1.63 times more than copper. It is very important that aluminum is less scarce than copper.
For electrical purposes, aluminum containing no more than 0.5% impurities, grade A1, is used. Even more pure AB00 aluminum (no more than 0.03% impurities) is used for the manufacture of aluminum foil, electrodes and housings of electrolytic capacitors. Aluminum of the highest purity AB0000 has an impurity content of no more than 0.004%. Additives of Ni, Si, Zn or Fe at a content of 0.5% reduce the γ of annealed aluminum by no more than 2-3%. A more noticeable effect is exerted by Cu, Ag and Mg impurities, which, at the same mass content, reduce γ aluminum by 5-10%. Ti and Mn greatly reduce the electrical conductivity of aluminum.
Aluminum oxidizes very actively and becomes covered with a thin oxide film with high electrical resistance. This film protects the metal from further corrosion.
Aluminum alloys have increased mechanical strength. An example of such an alloy is aldrey , containing 0.3-0.5% Mg, 0.4-0.7% Si and 0.2-0.3% Fe. In aldrey, a Mg 2 Si compound is formed, which imparts high mechanical properties to the alloy.
2.4.3. Iron
Iron (steel), as the cheapest and most accessible metal, which also has high mechanical strength, is of great interest for use as a conductor material. However, even pure iron has a significantly higher resistivity compared to copper and aluminum; ρ steel, i.e. iron mixed with carbon and other elements is even higher. Ordinary steel has low corrosion resistance: even at normal temperatures, especially in conditions of high humidity, it quickly rusts; As the temperature rises, the corrosion rate increases sharply. Therefore, the surface of steel wires must be protected by a layer of more resistant material. Zinc coating is usually used for this purpose.
In some cases, to reduce the consumption of non-ferrous metals, the so-called bimetal . It is steel coated on the outside with a layer of copper, with both metals connected to each other firmly and continuously.
2.4.4. Sodium
Sodium metal is a very promising conductor material. Sodium can be obtained by electrolysis of molten sodium chloride NaCl in virtually unlimited quantities. From a comparison of the properties of sodium with the properties of other conductor metals, it is clear that the resistivity of sodium is approximately 2.8 times greater than ρ of copper and 1.7 times greater than ρ of aluminum, but due to the extremely low density of sodium (its density is almost 9 times less than the density of copper), a wire made of sodium for a given conductivity per unit length should be significantly lighter than a wire made of any other metal. However, sodium is extremely active chemically (it oxidizes intensely in air and reacts violently with water), which is why the sodium wire must be protected with a sealing sheath. The sheath must give the wire the necessary mechanical strength, since sodium is very soft and has a low tensile strength during deformation.
2.5. Superconductors and cryoconductors
As already noted, as the temperature decreases, the resistivity of metals decreases. Of particular interest is the question of the electrical conductivity of metals at very low temperatures approaching absolute zero. The disappearance of electrical resistance, i.e. the appearance of practically infinite electrical conductivity of a material is called superconductivity , and the temperature, upon cooling to which the substance transitions to the superconducting state, is superconducting transition temperature T s. The transition to the superconducting state is reversible: when the temperature rises to T c, superconductivity is destroyed and the material goes into the normal state, acquiring a final value of specific conductivity γ. Currently, 27 simple (pure metals) and more than a thousand complex (alloys and chemical compounds) are known.
At the same time, some substances, including the best conductor materials such as silver and copper, at the lowest temperatures currently achieved (on the order of thousandths of a Kelvin; according to the third law of thermodynamics, absolute zero temperature is fundamentally unattainable) into a superconducting state failed. It is interesting to note that superconductors can be not only compounds and alloys of metals that have superconductivity, but also compounds of such elements with non-superconducting ones and even compounds whose molecules contain exclusively atoms of elements that are not superconducting.
In addition to superconducting electromagnets, one can note the possibility of using superconductors to create electrical machines, transformers and similar devices of low mass and dimensions, but with high efficiency; power transmission lines of very high power over long distances; waveguides with particularly low attenuation; energy storage devices, etc.
In addition to the phenomenon of superconductivity, the phenomenon of cryoconductivity , i.e. the achievement by some metals of very low specific conductivity at cryogenic temperatures (but higher than the superconducting transition temperature, if a given metal belongs to superconductors at all. Materials with particularly favorable properties for use as conductors at cryogenic temperatures are called cryoconductors or hyperconductors .
The very small, but still finite value of the resistivity of a cryoconductor at its operating temperature limits the permissible current density in it, although this density can be much higher than in conventional conductors. Cryoconductors, whose resistivity changes smoothly when the temperature changes over a wide range, without jumps, cannot be used in a number of devices whose operation is based on the trigger effect of the appearance and destruction of superconductivity. However, the use of cryoconductors in electrical machines, devices, cables, etc. has its advantages, and quite significant ones at that. Thus, if liquid helium is used as a cooling agent in superconducting devices, the operating temperature of cryoconductors is achieved by using higher-boiling and cheaper coolants: liquid hydrogen or even liquid nitrogen. This greatly simplifies and reduces the cost of implementation and operation of the device. In addition, in a superconducting device, such as an electromagnet, in which a strong current passes through the windings, a large amount of magnetic field energy accumulates. If, due to an accidental increase in temperature or magnetic induction, at least in a small section of the superconducting circuit, superconductivity is destroyed, suddenly the a large number of energy, which could cause a serious accident. In the case of a cryoconductor circuit, an increase in temperature will only cause a gradual increase in the resistance of this circuit without the effect of an explosion.
In all cases, the production of cryoconductor materials requires high purity of the metal and the absence of hardening. The harmful effect of impurities and cold hardening on the ρ of metals at cryogenic temperatures is much stronger than at normal temperatures. Cryoconductors can be successfully used for windings of electrical machines and transformers, for conductive cable cores, etc.
2.6. High resistance alloys
In addition to high resistance, such materials require high stability ρ over time, low TKρ and low thermal emf coefficient. paired this alloy with copper. It is desirable that such alloys be cheap and, if possible, do not contain scarce components.
2.6.1. Manganin
This is the most typical and widely used alloy for reference resistors. Its approximate composition: Cu - 85%, Mn - 12% and Ni - 3%; the name comes from the presence of manganese in it; The yellowish color is due to the high copper content. ρ manganin 0.42-0.48 µOhm∙m, thermo-emf coefficient. paired with copper only 1-2 µV/K, α ρ is very small. The maximum long-term permissible operating temperature is not more than 200°C.
2.6.2. Constantan
An alloy containing about 60% copper and 40% nickel; this composition corresponds to the minimum α ρ in the Cu-Ni system at quite high valueρ. The name constantan is explained by the significant constancy of ρ with temperature changes. The heat resistance of constantan is higher than that of manganin, and the mechanical properties are similar. A significant difference between the latter is its high thermal emf. paired with copper and iron. Widespread use of constantan is hampered by great content expensive and scarce nickel.
2.6.3. Iron-based alloys
Alloys of the Fe – Ni – Cr system are called nichromes or (with increased iron content) ferronichromes ; alloys of the Fe – Cr – Al system are called fechrals And limping . Nichromes are very technologically advanced: they can be easily drawn into thin wire or tape, they have a high operating temperature. However, like costantan, they have a high nickel content. Nichromes are used as electric heating elements.
Chromium-aluminum alloys are much cheaper than nichrome, but these alloys are less technologically advanced, harder and more brittle. They are mainly used for high power electric heating devices.
2.7. Refractory metals
Refractory metals include metals with a melting point exceeding 1700°C. As a rule, they are chemically stable at low temperatures, but become active at elevated temperatures. Their operation at high temperatures can be provided in an atmosphere of inert gases or in a vacuum. In dense form, these metals are most often obtained by powder metallurgy methods - pressing and sintering. Electronic or laser beam melting, zone cleaning, plasma processing, etc. are beginning to spread in electronic technology. Mechanical processing of these materials is difficult and often requires heating.
2.7.1. Tungsten
Extremely heavy, hard metal gray. Of all the metals, tungsten has the highest melting point (3380°C). It is extracted from ores of various compositions, the most famous of which are wolframite (FeWO 4 + MnWO 4) and scheelite (CaWO 4) by complex chemical treatment. Tungsten is characterized by weak mechanical cohesion of crystals, therefore, with a granular structure, relatively thick tungsten products are very fragile and easily break. As a result of mechanical processing by forging and drawing, tungsten acquires a fibrous structure and its fracture is very difficult. This explains the flexibility of thin tungsten filaments.
Tungsten is used to make filaments of incandescent lamps, as well as electrodes, heaters, springs and hooks in electron tubes, X-ray tubes, etc. Due to the refractoriness and large mechanical strength, tungsten can operate at high temperatures (more than 2000°C), but only in a high vacuum or in an inert gas atmosphere, because when heated to a temperature of several hundred degrees in the presence of oxygen, it strongly oxidizes.
2.7.2. Molybdenum
This metal is close in appearance and processing technology to tungsten. The most important industrial molybdenum ore is molybdenite MoS 2 . Molybdenum is used in electric vacuum technology at lower temperatures than tungsten; heated parts made of molybdenum must operate in a vacuum or reducing atmosphere.
2.7.3. Tantalum
It is obtained from a less common ore - tantalite Fe(TaO 3) 2 using powder metallurgy methods, like tungsten and molybdenum. Its main difference is only that the sintering process is carried out in vacuum furnaces, because tantalum tends to absorb gases, causing it to become brittle. Tantalum is characterized by high ductility even at room temperature. Tantalum is classified as a superconductor and is used in the manufacture of anodes and grids of generator lamps, etc.
2.7.4. Titanium
Relatively light metal, used in electrovacuum technology due to its good mechanical properties. The main minerals containing titanium are rutile and ilmenium. Titanium is produced using powder metallurgy methods. It is used not only as a structural material, but also for powder coatings of molybdenum and tungsten anodes and grids of generator lamps. It is also used to make integrated circuit resistors.
2.7.5. Rhenium
One of the rare, very heavy metals, with a melting point close to tungsten. Rhenium is distinguished by a rare combination of properties that satisfy most of the requirements of electrovacuum technology. In a hydrogen atmosphere and in a humid environment, it evaporates to a lesser extent than tungsten. A valuable feature of rhenium is its lower degree of interaction, compared with tungsten, at high temperatures with aluminum oxide, from which insulating tubes for heated cathodes of direct filament and grids of some types of lamps are made.
2.8. Noble metals
Noble metals include gold, silver, platinum and platinum group metals (ruthenium Ru, rhodium Rh, palladium Pd, osmium Os and iridium Ir). These metals are called noble for their beautiful appearance and high chemical resistance. They are used as conductors and contacts for corrosion-resistant coatings and photocell electrodes. Silver is also used for direct application to dielectrics as plating in the production of ceramic and mica capacitors.
2.9. Non-metallic conductors
Among solid non-metallic conductor materials highest value have carbon-based materials. Coal is used to make brushes for electric machines, electrodes for spotlights, and electrodes for arc electric ovens and electrolytic baths, anodes of galvanic cells. Coal powders are used in microphones; high-resistance resistors and arresters for telephone networks are made from coal.
Carbon black, graphite and anthracite can be used as raw materials for the production of electric carbon products. Natural graphite is one of the modifications of pure carbon with a layered structure with great anisotropy of both electrical and mechanical properties. Soots are finely dispersed carbon with admixtures of layered substances. Varnishes that contain soot as a pigment have low resistivity and can be used to equalize the electric field in electric machines high voltage.