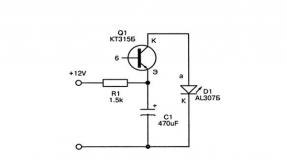
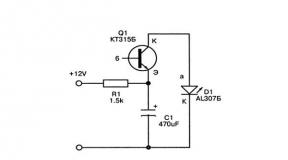
The membrane potential is 35. Membrane potential
": The resting potential is an important phenomenon in the life of all cells in the body, and it is important to know how it is formed. However, this is a complex dynamic process, difficult to comprehend in its entirety, especially for junior students (biological, medical and psychological specialties) and unprepared readers. However, when considered point by point, it is quite possible to understand its main details and stages. The work introduces the concept of the resting potential and highlights the main stages of its formation using figurative metaphors that help to understand and remember the molecular mechanisms of the formation of the resting potential.
Membrane transport structures - sodium-potassium pumps - create the prerequisites for the emergence of a resting potential. These prerequisites are the difference in ion concentration on the inner and outer sides of the cell membrane. The difference in sodium concentration and the difference in potassium concentration manifest themselves separately. An attempt by potassium ions (K+) to equalize their concentration on both sides of the membrane leads to its leakage from the cell and the loss of positive electrical charges along with them, due to which the overall negative charge of the inner surface of the cell is significantly increased. This "potassium" negativity constitutes the majority of the resting potential (−60 mV on average), and a smaller portion (−10 mV) is the "exchange" negativity caused by the electrogenicity of the ion exchange pump itself.
Let's take a closer look.
Why do we need to know what resting potential is and how it arises?
Do you know what “animal electricity” is? Where do “biocurrents” come from in the body? How can a living cell located in an aquatic environment turn into an “electric battery” and why does it not immediately discharge?
These questions can only be answered if we know how the cell creates its own difference. electrical potentials(resting potential) across the membrane.
It is quite obvious that in order to understand how the nervous system works, it is necessary to first understand how its individual nerve cell, the neuron, works. The main thing that underlies the work of a neuron is the movement of electrical charges through its membrane and, as a result, the appearance of electrical potentials on the membrane. We can say that a neuron, preparing for its nervous work, first stores energy in electrical form, and then uses it in the process of conducting and transmitting nervous excitation.
Thus, our very first step to studying the work nervous system- is to understand how the electrical potential appears on the membrane of nerve cells. This is what we will do, and we will call this process formation of the resting potential.
Definition of the concept of “resting potential”
Normally, when a nerve cell is at physiological rest and ready to work, it has already experienced a redistribution of electrical charges between the inner and outer sides of the membrane. Due to this it arose electric field, and an electric potential appeared on the membrane - resting membrane potential.
Thus, the membrane becomes polarized. This means that it has different electrical potentials on the outer and inner surfaces. The difference between these potentials is quite possible to register.
This can be verified if a microelectrode connected to a recording unit is inserted into the cell. As soon as the electrode gets inside the cell, it instantly acquires some constant electronegative potential with respect to the electrode located in the fluid surrounding the cell. The value of the intracellular electrical potential in nerve cells and fibers, for example, the giant nerve fibers of the squid, at rest is about −70 mV. This value is called the resting membrane potential (RMP). At all points of the axoplasm this potential is almost the same.
Nozdrachev A.D. and others. Beginnings of physiology.
A little more physics. Macroscopic physical bodies, as a rule, are electrically neutral, i.e. they contain both positive and negative charges in equal quantities. You can charge a body by creating an excess of charged particles of one type in it, for example, by friction against another body, in which an excess of charges of the opposite type is formed. Considering the presence of an elementary charge ( e), the total electric charge of any body can be represented as q= ±N× e, where N is an integer.
Resting potential- this is the difference in electrical potentials present on the inner and outer sides of the membrane when the cell is in a state of physiological rest. Its value is measured from inside the cell, it is negative and averages −70 mV (millivolts), although it can vary in different cells: from −35 mV to −90 mV.
It is important to consider that in the nervous system, electrical charges are not represented by electrons, as in ordinary metal wires, but by ions - chemical particles that have an electrical charge. In general, in aqueous solutions, it is not electrons, but ions that move in the form of electric current. Therefore, all electrical currents in cells and their environment are ion currents.
So, the inside of the cell at rest is negatively charged, and the outside is positively charged. This is characteristic of all living cells, with the possible exception of red blood cells, which, on the contrary, are negatively charged on the outside. More specifically, it turns out that positive ions (Na + and K + cations) will predominate outside the cell around the cell, and negative ions (anions of organic acids that are not able to move freely through the membrane, like Na + and K +) will prevail inside.
Now we just have to explain how everything turned out this way. Although, of course, it is unpleasant to realize that all our cells except red blood cells only look positive on the outside, but on the inside they are negative.
The term “negativity,” which we will use to characterize the electrical potential inside the cell, will be useful to us to easily explain changes in the level of the resting potential. What is valuable about this term is that the following is intuitively clear: the greater the negativity inside the cell, the lower the potential is shifted to the negative side from zero, and the less negativity, the closer the negative potential is to zero. This is much easier to understand than to understand every time what exactly the expression “potential increases” means - an increase in absolute value (or “modulo”) will mean a shift of the resting potential down from zero, and simply an “increase” means a shift of the potential up to zero. The term "negativity" does not create such problems of ambiguity of understanding.
The essence of the formation of the resting potential
Let's try to figure out where the electric charge of nerve cells comes from, although no one rubs them, as physicists do in their experiments with electric charges.
Here one of the logical traps awaits the researcher and student: the internal negativity of the cell does not arise due to the appearance of extra negative particles(anions), but, on the contrary, due to loss of a certain amount of positive particles(cations)!
So where do positively charged particles go from the cell? Let me remind you that these are sodium ions - Na + - and potassium - K + that have left the cell and accumulated outside.
The main secret of the appearance of negativity inside the cell
Let’s immediately reveal this secret and say that the cell loses some of its positive particles and becomes negatively charged due to two processes:
- first, she exchanges “her” sodium for “foreign” potassium (yes, some positive ions for others, equally positive);
- then these “replaced” positive potassium ions leak out of it, along with which positive charges leak out of the cell.
We need to explain these two processes.
The first stage of creating internal negativity: exchange of Na + for K +
Proteins are constantly working in the membrane of a nerve cell. exchanger pumps(adenosine triphosphatases, or Na + /K + -ATPases) embedded in the membrane. They exchange the cell’s “own” sodium for external “foreign” potassium.
But when one positive charge (Na +) is exchanged for another identical positive charge (K +), no deficiency of positive charges can arise in the cell! Right. But, nevertheless, due to this exchange, very few sodium ions remain in the cell, because almost all of them have gone outside. And at the same time, the cell is overflowing with potassium ions, which were pumped into it by molecular pumps. If we could taste the cytoplasm of the cell, we would notice that as a result of the work of the exchange pumps, it turned from salty to bitter-salty-sour, because the salty taste of sodium chloride was replaced by the complex taste of a rather concentrated solution of potassium chloride. In the cell, the potassium concentration reaches 0.4 mol/l. Solutions of potassium chloride in the range of 0.009-0.02 mol/l have a sweet taste, 0.03-0.04 - bitter, 0.05-0.1 - bitter-salty, and starting from 0.2 and above - a complex taste consisting of salty, bitter and sour.
The important thing here is that exchange of sodium for potassium - unequal. For every cell given three sodium ions she gets everything two potassium ions. This results in the loss of one positive charge with each ion exchange event. So already at this stage, due to unequal exchange, the cell loses more “pluses” than it receives in return. In electrical terms, this amounts to approximately −10 mV of negativity within the cell. (But remember that we still need to find an explanation for the remaining −60 mV!)
To make it easier to remember the operation of exchanger pumps, we can figuratively put it this way: “The cell loves potassium!” Therefore, the cell drags potassium towards itself, despite the fact that it is already full of it. And therefore, it exchanges it unprofitably for sodium, giving 3 sodium ions for 2 potassium ions. And therefore it spends ATP energy on this exchange. And how he spends it! Up to 70% of a neuron’s total energy expenditure can be spent on the operation of sodium-potassium pumps. (That's what love does, even if it's not real!)
By the way, it is interesting that the cell is not born with a ready-made resting potential. She still needs to create it. For example, during differentiation and fusion of myoblasts, their membrane potential changes from −10 to −70 mV, i.e. their membrane becomes more negative - polarized during the process of differentiation. And in experiments on multipotent mesenchymal stromal cells of human bone marrow, artificial depolarization, counteracting the resting potential and reducing cell negativity, even inhibited (depressed) cell differentiation.
Figuratively speaking, we can put it this way: By creating a resting potential, the cell is “charged with love.” This is love for two things:
- the cell's love for potassium (therefore the cell forcibly drags it towards itself);
- potassium's love for freedom (therefore potassium leaves the cell that has captured it).
We have already explained the mechanism of saturating the cell with potassium (this is the work of exchange pumps), and the mechanism of potassium leaving the cell will be explained below, when we move on to describing the second stage of creating intracellular negativity. So, the result of the activity of membrane ion exchanger pumps at the first stage of the formation of the resting potential is as follows:
- Sodium (Na+) deficiency in the cell.
- Excess potassium (K+) in the cell.
- The appearance of a weak electric potential (−10 mV) on the membrane.
We can say this: at the first stage, membrane ion pumps create a difference in ion concentrations, or a concentration gradient (difference), between the intracellular and extracellular environment.
Second stage of creating negativity: leakage of K+ ions from the cell
So, what begins in the cell after its membrane sodium-potassium exchanger pumps work with ions?
Due to the resulting sodium deficiency inside the cell, this ion strives to rush inside: dissolved substances always strive to equalize their concentration throughout the entire volume of the solution. But sodium does this poorly, since sodium ion channels are usually closed and open only under certain conditions: under the influence of special substances (transmitters) or when the negativity in the cell decreases (membrane depolarization).
At the same time, there is an excess of potassium ions in the cell compared to the external environment - because the membrane pumps forcibly pumped it into the cell. And he, also trying to equalize his concentration inside and outside, strives, on the contrary, get out of the cage. And he succeeds!
Potassium ions K + leave the cell under the influence of a chemical gradient of their concentration on different sides of the membrane (the membrane is much more permeable to K + than to Na +) and carry away positive charges with them. Because of this, negativity grows inside the cell.
It is also important to understand that sodium and potassium ions do not seem to “notice” each other, they react only “to themselves.” Those. sodium reacts to the same sodium concentration, but “does not pay attention” to how much potassium is around. Conversely, potassium only responds to potassium concentrations and “ignores” sodium. It turns out that to understand the behavior of ions, it is necessary to separately consider the concentrations of sodium and potassium ions. Those. it is necessary to separately compare the concentration of sodium inside and outside the cell and separately - the concentration of potassium inside and outside the cell, but it makes no sense to compare sodium with potassium, as is sometimes done in textbooks.
According to the law of equalization of chemical concentrations, which operates in solutions, sodium “wants” to enter the cell from the outside; it is also drawn there by electrical force (as we remember, the cytoplasm is negatively charged). He wants to, but he can’t, because the membrane is in normal condition misses it poorly. Sodium ion channels present in the membrane are normally closed. If, nevertheless, a little of it comes in, then the cell immediately exchanges it for external potassium using its sodium-potassium exchanger pumps. It turns out that sodium ions pass through the cell as if in transit and do not stay in it. Therefore, sodium in neurons is always in short supply.
But potassium can easily leave the cell to the outside! The cage is full of him, and she can’t hold him. It exits through special channels in the membrane - "potassium leak channels", which are normally open and release potassium.
K+ leakage channels are constantly open at normal values membrane potential rest and exhibit bursts of activity during shifts in membrane potential, which last several minutes and are observed at all potential values. An increase in K+ leakage currents leads to hyperpolarization of the membrane, while their suppression leads to depolarization. ...However, the existence of a channel mechanism responsible for leakage currents remained in question for a long time. Only now has it become clear that potassium leakage is a current through special potassium channels.
Zefirov A.L. and Sitdikova G.F. Ion channels of an excitable cell (structure, function, pathology).
From chemical to electrical
And now - once again the most important thing. We must consciously move away from movement chemical particles to the movement electric charges.
Potassium (K+) is positively charged, and therefore, when it leaves the cell, it carries out not only itself, but also a positive charge. Behind it, “minuses” - negative charges - stretch from inside the cell to the membrane. But they cannot leak through the membrane - unlike potassium ions - because... there are no suitable ion channels for them, and the membrane does not allow them to pass through. Remember about the −60 mV of negativity that remains unexplained by us? This is the very part of the resting membrane potential that is created by the leakage of potassium ions from the cell! And this is a large part of the resting potential.
There is even a special name for this component of the resting potential - concentration potential. Concentration potential - this is part of the resting potential created by the deficiency of positive charges inside the cell, formed due to the leakage of positive potassium ions from it.
Well, now a little physics, chemistry and mathematics for lovers of precision.
Electrical forces are related to chemical forces according to the Goldmann equation. Its special case is the simpler Nernst equation, the formula of which can be used to calculate the transmembrane diffusion potential difference based on different concentrations of ions of the same type on different sides of the membrane. So, knowing the concentration of potassium ions outside and inside the cell, we can calculate the potassium equilibrium potential E K:

Where E k - equilibrium potential, R- gas constant, T- absolute temperature, F- Faraday's constant, K + ext and K + int - concentrations of K + ions outside and inside the cell, respectively. The formula shows that to calculate the potential, the concentrations of ions of the same type - K + - are compared with each other.
More precisely, the final value of the total diffusion potential, which is created by the leakage of several types of ions, is calculated using the Goldman-Hodgkin-Katz formula. It takes into account that the resting potential depends on three factors: (1) the polarity of the electric charge of each ion; (2) membrane permeability R for each ion; (3) [concentrations of the corresponding ions] inside (internal) and outside the membrane (external). For the squid axon membrane at rest, the conductance ratio R K: PNa :P Cl = 1: 0.04: 0.45.
Conclusion
So, the resting potential consists of two parts:
- −10 mV, which are obtained from the “asymmetrical” operation of the membrane pump-exchanger (after all, it pumps more positive charges (Na +) out of the cell than it pumps back with potassium).
- The second part is potassium leaking out of the cell all the time, carrying away positive charges. His main contribution is: −60 mV. In total, this gives the desired −70 mV.
Interestingly, potassium will stop leaving the cell (more precisely, its input and output are equalized) only at a cell negative level of −90 mV. In this case, the chemical and electrical forces that push potassium through the membrane are equal, but direct it into opposite sides. But this is hampered by sodium constantly leaking into the cell, which carries with it positive charges and reduces the negativity for which potassium “fights.” And as a result, the cell maintains an equilibrium state at a level of −70 mV.
Now the resting membrane potential is finally formed.
Scheme of operation of Na + /K + -ATPase clearly illustrates the “asymmetrical” exchange of Na + for K +: pumping out excess “plus” in each cycle of the enzyme leads to negative charging of the inner surface of the membrane. What this video doesn't say is that the ATPase is responsible for less than 20% of the resting potential (−10 mV): the remaining "negativity" (−60 mV) comes from K ions leaving the cell through "potassium leak channels" +, seeking to equalize their concentration inside and outside the cell.
Literature
- Jacqueline Fischer-Lougheed, Jian-Hui Liu, Estelle Espinos, David Mordasini, Charles R. Bader, et. al.. (2001). Human Myoblast Fusion Requires Expression of Functional Inward Rectifier Kir2.1 Channels . J Cell Biol. 153 , 677-686;
- Liu J.H., Bijlenga P., Fischer-Lougheed J. et al. (1998). Role of an inward rectifier K+ current and of hyperpolarization in human myoblast fusion. J. Physiol. 510 , 467–476;
- Sarah Sundelacruz, Michael Levin, David L. Kaplan. (2008). Membrane Potential Controls Adipogenic and Osteogenic Differentiation of Mesenchymal Stem Cells. PLoS ONE. 3 , e3737;
- Pavlovskaya M.V. and Mamykin A.I. Electrostatics. Dielectrics and conductors in an electric field. Direct current / Electronic manual for the general course of physics. SPb: St. Petersburg State Electrotechnical University;
- Nozdrachev A.D., Bazhenov Yu.I., Barannikova I.A., Batuev A.S. and others. The beginnings of physiology: Textbook for universities / Ed. acad. HELL. Nozdracheva. St. Petersburg: Lan, 2001. - 1088 pp.;
- Makarov A.M. and Luneva L.A. Fundamentals of electromagnetism / Physics at a technical university. T. 3;
- Zefirov A.L. and Sitdikova G.F. Ion channels of an excitable cell (structure, function, pathology). Kazan: Art Cafe, 2010. - 271 p.;
- Rodina T.G. Sensory analysis food products. Textbook for university students. M.: Academy, 2004. - 208 pp.;
- Kolman, J. and Rehm, K.-G. Visual biochemistry. M.: Mir, 2004. - 469 pp.;
- Shulgovsky V.V. Basics of neurophysiology: Tutorial for university students. M.: Aspect Press, 2000. - 277 pp..
Resting membrane potential (MPP) or resting potential (PP) is the potential difference of a resting cell between the inner and outer sides of the membrane. The inner side of the cell membrane is negatively charged relative to the outer. Taking the potential of the external solution as zero, the MPP is written with a minus sign. Magnitude MPP depends on the type of tissue and varies from -9 to -100 mV. Therefore, in a state of rest the cell membrane polarized. A decrease in the MPP value is called depolarization, increase - hyperpolarization, restoring the original value MPP-repolarization membranes.
Basic provisions of the membrane theory of origin MPP boil down to the following. In the resting state, the cell membrane is highly permeable to K + ions (in some cells and for SG), less permeable to Na + and practically impermeable to intracellular proteins and other organic ions. K+ ions diffuse out of the cell along a concentration gradient, and non-penetrating anions remain in the cytoplasm, providing the appearance of a potential difference across the membrane.
The resulting potential difference prevents the exit of K+ from the cell and at a certain value, an equilibrium occurs between the exit of K+ along the concentration gradient and the entry of these cations along the resulting electrical gradient. The membrane potential at which this equilibrium is achieved is called equilibrium potential. Its value can be calculated from the Nernst equation:
10 In nerve fibers, signals are transmitted by action potentials, which are rapid changes in membrane potential that propagate rapidly along the nerve fiber membrane. Each action potential begins with a rapid shift of the resting potential from a normal negative value to a positive value, then it returns almost as quickly to a negative potential. When a nerve signal is conducted, the action potential moves along the nerve fiber until it ends. The figure shows the changes that occur at the membrane during an action potential, with positive charges moving into the fiber at the beginning and positive charges returning outward at the end. The lower part of the figure graphically represents the successive changes in membrane potential over a period of several 1/10,000 sec, illustrating the explosive onset of the action potential and an almost equally rapid recovery. Rest stage. This stage is represented by the resting membrane potential, which precedes the action potential. The membrane is polarized during this stage due to the presence of a negative membrane potential of -90 mV. Depolarization phase. At this time, the membrane suddenly becomes highly permeable to sodium ions, allowing large numbers of positively charged sodium ions to diffuse into the axon. The normal polarized state of -90 mV is immediately neutralized by the incoming positively charged sodium ions, causing the potential to rapidly increase in the positive direction. This process is called depolarization. In large nerve fibers, a significant excess of incoming positive sodium ions usually causes the membrane potential to “jump” beyond the zero level, becoming slightly positive. In some smaller fibers, as in most neurons of the central nervous system, the potential reaches the zero level without “jumping” over it. Repolarization phase. Within a few fractions of a millisecond after a sharp increase in the permeability of the membrane to sodium ions, sodium channels begin to close and potassium channels begin to open. As a result, rapid outward diffusion of potassium ions restores the normal negative resting membrane potential. This process is called membrane repolarization. action potential To more fully understand the factors that cause depolarization and repolarization, it is necessary to study the characteristics of two other types of transport channels in the nerve fiber membrane: electrically gated sodium and potassium channels. Electrogated sodium and potassium channels. An electrically controlled sodium channel is a necessary participant in the processes of depolarization and repolarization during the development of an action potential in the nerve fiber membrane. The electrically gated potassium channel also plays an important role in increasing the rate of membrane repolarization. Both types of electrically controlled channels exist in addition to the Na+/K+ pump and K*/Na+ leakage channels. Electrically controlled sodium channel. The top part of the figure shows an electrically driven sodium channel in three different states. This channel has two gates: one near the outer part of the channel, which is called the activation gate, the other - near the inner part of the channel, which is called the inactivation gate. The upper left part of the figure shows the resting state of this gate when the resting membrane potential is -90 mV. Under these conditions, the activation gate is closed and prevents sodium ions from entering the fiber. Sodium channel activation. When the resting membrane potential shifts towards less negative values, rising from -90 mV towards zero, at a certain level (usually between -70 and -50 mV) a sudden conformational change occurs in the activation gate, resulting in it moving into a completely open state . This state is called the activated state of the channel, in which sodium ions can freely enter the fiber through it; in this case, the sodium permeability of the membrane increases in the range from 500 to 5000 times. Inactivation of the sodium channel. The upper right part of the figure shows the third state of the sodium channel. The increase in potential that opens the activation gate closes the inactivation gate. However, the inactivation gate closes within a few tenths of a millisecond after the activation gate opens. This means that the conformational change that leads to the closing of the inactivation gate is a slower process than the conformational change that opens the activation gate. As a result, a few tenths of a millisecond after the opening of the sodium channel, the inactivation gate closes, and sodium ions can no longer penetrate into the fiber. From this moment, the membrane potential begins to return to the resting level, i.e. the repolarization process begins. There is another important characteristic of the sodium channel inactivation process: the inactivation gate does not re-open until the membrane potential returns to a value equal to or close to the level of the original resting potential. In this regard, re-opening of sodium channels is usually impossible without prior repolarization of the nerve fiber.
13The mechanism for conducting excitation along nerve fibers depends on their type. There are two types of nerve fibers: myelinated and unmyelinated. Metabolic processes in unmyelinated fibers do not provide rapid compensation for energy expenditure. The spread of excitation will occur with gradual attenuation - with decrement. Decremental behavior of excitation is characteristic of a low-organized nervous system. Excitation propagates due to small circular currents that arise into the fiber or into the surrounding liquid. A potential difference arises between excited and unexcited areas, which contributes to the emergence of circular currents. The current will spread from the “+” charge to the “-”. At the point where the circular current exits, the permeability of the plasma membrane for Na ions increases, resulting in depolarization of the membrane. A potential difference again arises between the newly excited area and the neighboring unexcited one, which leads to the emergence of circular currents. The excitation gradually covers neighboring areas of the axial cylinder and thus spreads to the end of the axon. In myelin fibers, thanks to the perfection of metabolism, excitation passes without fading, without decrement. Due to the large radius of the nerve fiber due to the myelin sheath, electric current can enter and exit the fiber only in the area of interception. When stimulation is applied, depolarization occurs in the area of interception A, and the neighboring interception B is polarized at this time. Between the interceptions, a potential difference arises, and circular currents appear. Due to circular currents, other interceptions are excited, while the excitation spreads saltatory, jumpwise from one interception to another. There are three laws for the conduction of stimulation along a nerve fiber. Law of anatomical and physiological integrity. Conduction of impulses along a nerve fiber is possible only if its integrity is not compromised. Law of isolated conduction of excitation. There are a number of features of the spread of excitation in peripheral, pulpal and non-pulpate nerve fibers. In peripheral nerve fibers, excitation is transmitted only along the nerve fiber, but is not transmitted to neighboring ones, which are located in the same nerve trunk. In the pulpy nerve fibers, the myelin sheath plays the role of an insulator. Due to myelin, the resistivity increases and the electrical capacitance of the sheath decreases. In non-pulp nerve fibers, excitation is transmitted in isolation. The law of two-way conduction of excitation. The nerve fiber conducts nerve impulses in two directions - centripetal and centrifugal.
14 Synapses - this is a specialized structure that ensures the transmission of a nerve impulse from a nerve fiber to an effector cell - a muscle fiber, neuron or secretory cell.
Synapses– these are the junctions of the nerve process (axon) of one neuron with the body or process (dendrite, axon) of another nerve cell (intermittent contact between nerve cells).
All structures that provide signal transmission from one nerve structure to another - synapses .
Meaning– transmits nerve impulses from one neuron to another => ensures the transmission of excitation along the nerve fiber (signal propagation).
A large number of synapses provides a large area for information transfer.
Synapse structure:
1. Presynaptic membrane- belongs to the neuron from which the signal is transmitted.
2. Synaptic cleft, filled with liquid with a high content of Ca ions.
3. Postsynaptic membrane- belongs to the cells to which the signal is transmitted.
There is always a gap between neurons filled with interstitial fluid.
Depending on the density of the membranes, there are:
- symmetrical(with the same membrane density)
- asymmetrical(the density of one of the membranes is higher)
Presynaptic membrane covers the extension of the axon of the transmitting neuron.
Extension - synaptic button/synaptic plaque.
On the plaque - synaptic vesicles (vesicles).
On the inner side of the presynaptic membrane - protein/hexagonal lattice(necessary for the release of the mediator), which contains the protein - neurin . Filled with synaptic vesicles that contain mediator– a special substance involved in signal transmission.
The composition of the vesicle membrane includes - Stenin (protein).
Postsynaptic membrane covers the effector cell. Contains protein molecules that are selectively sensitive to the mediator of a given synapse, which ensures interaction.
These molecules are part of the channels of the postsynaptic membrane + enzymes (many) that can destroy the connection of the transmitter with the receptors.
Receptors of the postsynaptic membrane.
The postsynaptic membrane contains receptors that are related to the mediator of a given synapse.
Between them is snaptic fissure . It is filled with intercellular fluid, which has a large number of calcium. It has a number of structural features - it contains protein molecules that are sensitive to the mediator that transmits signals.
15 Synaptic conduction delay
It takes a certain amount of time for the excitation to spread along the reflex arc. This time consists of the following periods:
1. the period temporarily necessary for excitation of receptors (receptors) and for conducting excitation impulses along afferent fibers to the center;
2. the period of time required for the spread of excitation through the nerve centers;
3. the period of time required for the propagation of excitation along the efferent fibers to the working organ;
4. latent period of the working organ.
16 Inhibition plays an important role in the processing of information entering the central nervous system. This role is especially pronounced in presynaptic inhibition. It regulates the excitation process more precisely, since individual nerve fibers can be blocked by this inhibition. Hundreds and thousands of impulses can approach one excitatory neuron through different terminals. At the same time, the number of impulses reaching the neuron is determined by presynaptic inhibition. Inhibition of lateral pathways ensures the selection of significant signals from the background. Blockade of inhibition leads to widespread irradiation of excitation and convulsions, for example, when presynaptic inhibition by bicuculline is turned off.
Resting membrane potential
At rest on outside plasma membrane located thin layer positive charges, and on inside– negative. The difference between them is called resting membrane potential. If we assume the outer charge to be zero, then the charge difference between the outer and inner surfaces of most neurons turns out to be close to -65 mV, although it can vary from -40 to -80 mV in individual cells.
The occurrence of this charge difference is due to the unequal distribution of potassium, sodium and chlorine ions inside and outside the cell, as well as the greater permeability of the resting cell membrane only for potassium ions.
In excitable cells, the resting membrane potential (RMP) can vary greatly, and this ability is the basis for the occurrence of electrical signals. A decrease in the resting membrane potential, for example from -65 to -60 mV, is called depolarization , and an increase, for example, from -65 to -70 mV, – hyperpolarization .
If depolarization reaches a certain critical level, for example -55 mV, then the permeability of the membrane for sodium ions becomes maximum for a short time, they rush into the cell and, therefore, the transmembrane potential difference rapidly decreases to 0 and then becomes positive. This circumstance leads to the closure of sodium channels and the rapid release of potassium ions from the cell through channels intended only for them: as a result, the original value of the resting membrane potential is restored. These rapidly occurring changes in resting membrane potential are called action potential. The action potential is a driven electrical signal; it quickly spreads along the axon membrane to its very end, and does not change its amplitude anywhere.
Except action potentials in a nerve cell, due to changes in its membrane permeability, local or local signals may arise: receptor potential And postsynaptic potential. Their amplitude is significantly smaller than that of the action potential; in addition, it decreases significantly as the signal propagates. For this reason, local potentials cannot propagate across the membrane far from their point of origin.
The work of the sodium-potassium pump in the cell creates a high concentration of potassium ions, and in the cell membrane there is space for these ions open channels. Potassium ions leaving the cell along a concentration gradient increase the number of positive charges on the outer surface of the membrane. There are many large-molecular organic anions in the cell, and therefore the membrane turns out to be negatively charged from the inside. All other ions can pass through the resting membrane in very small quantities, their channels are mostly closed. Consequently, the resting potential owes its origin mainly to the flow of potassium ions from the cell .
| |
Electrical signals: input, combined, conductive and output
 Neurons come into contact with certain target cells, and the cytoplasm of the contacting cells does not connect and a synaptic gap always remains between them.
Neurons come into contact with certain target cells, and the cytoplasm of the contacting cells does not connect and a synaptic gap always remains between them.
The modern version of the neural theory connects certain parts of the nerve cell with the nature of the electrical signals that arise in them. A typical neuron has four morphologically defined regions: dendrites, soma, axon, and presynaptic axon terminal. When a neuron is excited, four types of electrical signals appear in it sequentially: input, combined, conductive and output(Fig. 3.3). Each of these signals occurs only in a specific morphological region.
Input signals are either receptor, or postsynaptic potential. Receptor potential is formed in the endings of a sensitive neuron when a certain stimulus acts on them: stretching, pressure, light, Chemical substance and so on. The action of the stimulus causes the opening of certain ion channels in the membrane, and the subsequent flow of ions through these channels changes the initial value of the resting membrane potential; in most cases depolarization occurs. This depolarization is the receptor potential, its amplitude is proportional to the strength of the current stimulus.
The receptor potential can spread from the site of the stimulus along the membrane to a relatively short distance - the amplitude of the receptor potential decreases with distance from the site of the stimulus, and then the depolarizing shift will disappear altogether.
The second type of input signal is postsynaptic potential. It is formed on a postsynaptic cell after an excited presynaptic cell sends a neurotransmitter to it. Having reached the postsynaptic cell through diffusion, the mediator attaches to specific receptor proteins in its membrane, which causes the opening of ion channels. The resulting ion current through the postsynaptic membrane changes the initial value of the resting membrane potential - this shift is the postsynaptic potential.
In some synapses, such a shift represents depolarization and, if it reaches a critical level, the postsynaptic neuron is excited. In other synapses, a shift in the opposite direction occurs: the postsynaptic membrane is hyperpolarized: the value of the membrane potential becomes larger and it becomes more difficult to reduce it to a critical level of depolarization. It is difficult to excite such a cell; it is inhibited. Thus, the depolarizing postsynaptic potential is exciting, and hyperpolarizing – braking. Accordingly, the synapses themselves are divided into excitatory (causing depolarization) and inhibitory (causing hyperpolarization).
Regardless of what happens on the postsynaptic membrane: depolarization or hyperpolarization, the magnitude of postsynaptic potentials is always proportional to the number of transmitter molecules acting, but usually their amplitude is small. Just like the receptor potential, they spread along the membrane over a very short distance, i.e. also relate to local potentials.
Thus, input signals are represented by two types of local potentials, receptor and postsynaptic, and these potentials arise in strictly defined areas of the neuron: either in sensory endings or in synapses. Sensory endings belong to sensory neurons, where the receptor potential arises under the influence of external stimuli. For interneurons, as well as for efferent neurons, only the postsynaptic potential can be the input signal.
Combined signal can occur only in a region of the membrane where there are a sufficient number of ion channels for sodium. In this regard, the ideal object is the axon hillock - the place where the axon departs from the cell body, since it is here that the density of channels for sodium is highest in the entire membrane. Such channels are potential-dependent, i.e. open only when the initial value of the resting potential reaches a critical level. The typical resting potential for the average neuron is approximately -65 mV, and the critical level of depolarization corresponds to approximately -55 mV. Therefore, if it is possible to depolarize the membrane of the axon hillock from -65 mV to -55 mV, then an action potential will arise there.
Input signals are capable of depolarizing the membrane, i.e. either postsynaptic potentials or receptor potentials. In the case of receptor potentials, the place of origin of the combined signal is the node of Ranvier closest to the sensitive endings, where depolarization to a critical level is most likely. Each sensory neuron has many endings, which are branches of one process. And, if in each of these endings, during the action of a stimulus, a very small amplitude receptor potential arises and spreads to the node of Ranvier with a decrease in amplitude, then it is only a small part of the total depolarizing shift. From each sensitive ending, these small receptor potentials move at the same time to the nearest node of Ranvier, and in the area of the interception they are all summed up. If the total amount of depolarizing shift is sufficient, an action potential will arise at the interception.
Postsynaptic potentials arising on dendrites are as small as receptor potentials and also decrease as they propagate from the synapse to the axon hillock, where an action potential can arise. In addition, inhibitory hyperpolarizing synapses may be in the way of the propagation of postsynaptic potentials throughout the cell body, and therefore the possibility of depolarization of the axon hillock membrane by 10 mV seems unlikely. However, this result is regularly achieved as a result of the summation of many small postsynaptic potentials that arise simultaneously at numerous synapses formed by the dendrites of the neuron with the axon terminals of presynaptic cells.
Thus, the combined signal arises, as a rule, as a result of the summation of simultaneously formed numerous local potentials. This summation occurs in the place where there are especially many voltage-gated channels and therefore the critical level of depolarization is more easily achieved. In the case of integration of postsynaptic potentials, such a place is the axon hillock, and the summation of receptor potentials occurs in the node of Ranvier closest to the sensory endings (or the area of the unmyelinated axon close to them). The area where the combined signal occurs is called integrative or trigger.
The accumulation of small depolarizing shifts is transformed with lightning speed in the integrative zone into an action potential, which is the maximum electrical potential of the cell and occurs according to the “all or nothing” principle. This rule must be understood in such a way that depolarization below a critical level does not bring any result, and when this level is reached, the maximum response is always revealed, regardless of the strength of the stimuli: there is no third option.
Conducting an action potential. The amplitude of the input signals is proportional to the strength of the stimulus or the amount of neurotransmitter released at the synapse - such signals are called gradual. Their duration is determined by the duration of the stimulus or the presence of the transmitter in the synaptic cleft. The amplitude and duration of the action potential do not depend on these factors: both of these parameters are entirely determined by the properties of the cell itself. Therefore, any combination of input signals, any type of summation, under the single condition of depolarization of the membrane to a critical value, causes the same standard pattern of action potential in the trigger zone. It always has the maximum amplitude for a given cell and approximately the same duration, no matter how many times the conditions causing it are repeated.
Having arisen in the integrative zone, the action potential quickly spreads along the axon membrane. This occurs due to the appearance of a local electric current. Since the depolarized section of the membrane turns out to be differently charged than its neighbor, an electric current arises between the polarly charged sections of the membrane. Under the influence of this local current, the neighboring area is depolarized to a critical level, which causes the appearance of an action potential in it. In the case of a myelinated axon, such a neighboring section of the membrane is the node of Ranvier closest to the trigger zone, then the next one, and the action potential begins to “jump” from one node to another at a speed reaching 100 m/s.
Different neurons may differ from each other in many ways, but the action potentials arising in them are very difficult, often impossible, to distinguish. This is a highly stereotypical signal in a variety of cells: sensory, interneurons, motor. This stereotypy indicates that the action potential itself does not contain any information about the nature of the stimulus that generated it. The strength of the stimulus is indicated by the frequency of action potentials that occur, and specific receptors and well-ordered interneuron connections determine the nature of the stimulus.
Thus, the action potential generated in the trigger zone quickly spreads along the axon to its end. This movement is associated with the formation of local electrical currents, under the influence of which the action potential appears anew in neighboring plot axon. The parameters of the action potential when carried along the axon do not change at all, which allows information to be transmitted without distortion. If the axons of several neurons find themselves in a common bundle of fibers, then excitation propagates along each of them separately.
Output signal addressed to another cell or to several cells at the same time and in the vast majority of cases represents the release of a chemical intermediary - a mediator. In the presynaptic endings of the axon, the pre-stored transmitter is stored in synaptic vesicles, which accumulate in special areas - active zones. When the action potential reaches the presynaptic terminal, the contents of the synaptic vesicles are emptied into the synaptic cleft by exocytosis.
Chemical mediators of information transmission can be different substances: small molecules, such as acetylcholine or glutamate, or fairly large peptide molecules - all of them are specially synthesized in the neuron for signal transmission. Once in the synaptic cleft, the transmitter diffuses to the postsynaptic membrane and attaches to its receptors. As a result of the connection of receptors with the transmitter, the ion current through the channels of the postsynaptic membrane changes, and this leads to a change in the value of the resting potential of the postsynaptic cell, i.e. an input signal arises in it - in in this case postsynaptic potential.
Thus, in almost every neuron, regardless of its size, shape and position in the neuron chain, four functional areas can be found: local receptive zone, integrative zone, signal conduction zone and output or secretory zone(Fig. 3.3).
Electric charge, like mass, is a fundamental property of substances. There are two types of charges, conventionally designated as positive and negative.
Every substance has an electrical charge, the value of which can be positive, negative or zero. For example, electrons are negatively charged and protons are positively charged. Since each atom contains one or more electrons and an equal number of protons, total number charges in a macroscopic object is extremely large, but in general such an object is not charged or has a small charge.
The electron charge is the smallest in absolute value.
Electric field. Coulomb's law
Every charged object creates an electric field in the space surrounding it. Electric field is a type of matter through which charged objects interact with each other. A test charge introduced into the electric field of another charge “feels” the presence of this field. It will be attracted to or repelled by the charge creating the electric field.
Coulomb's law
defines the electric force F acting between two point charges q 1 And q 2:
k- constant determined by the selected conditions; r- distance between charges.
According to Coulomb's law, a force acts in the direction of a line connecting two charges. The magnitude of the force acting on the charges is proportional to the size of each of the charges and inversely proportional to the square of the distance between them.
The electric field can be represented as power lines, showing the direction of electrical forces. These forces are directed away from the charge when it is positive and towards the charge when it is negative. If a positive charge is placed in an electric field, it experiences a force in the direction of the field. A negative charge is subject to a force opposite to the direction of the field.
Electric field characteristics
1) Electric field strength.
Every electric charge produces an electric field around itself. If another charge q enter into this field, then a force will act on it F, proportional q and electric field strength E:
The electric field strength E (or simply intensity) at any point is defined as the electric force F that acts on a positive charge q, placed at this point:
E is a vector quantity, that is, it has both magnitude and direction. The unit of measurement for voltage is volt per meter [V/m].
The principle of superposition (superposition) indicates that if an electric field is created by many charges, the total intensity will be determined by the addition of the intensities created by each charge, according to the rules of vector addition.
2) Electric potential. To move a charge against the electrical force acting on it, work must be done. This work does not depend on the path of movement of the charge in the electric field, but depends on the initial and final position of the charge.
If a charge is moved from one point to another against an electric force, its electrostatic potential energy increases. Electric potential at any point is equal to electrostatic potential energy W p, which has a positive charge q at this point: φ = W p /q (4).
We can also say that the electric potential at a point is equal to the work that must be done against the electric forces to move a positive charge from that point to a large distance where the electric field potential is zero. Electric potential is a scalar quantity and is measured in volts ( IN).
The electric field strength is the negative gradient of the electric potential - an indicator of the change in potential with distance x: E → = - dφ/dx. Using instruments, you can measure the potential difference, but not the field strength. The latter can be calculated using the dependence between E → and Δφ: where Δφ = E l- the distance between two electric field currents.
Resting membrane potential
Each cell converts some of its metabolic energy into electrostatic energy. The source of the cell's electric field is the plasma membrane. There is a potential difference between the inner and outer surfaces of the plasma membrane. This potential difference is called membrane potential .
Potential difference between internal and external environment cells can be measured directly and quite accurately. For this purpose, a microelectrode is used, which is a glass micropipette with a tip diameter of up to 1 µm filled with concentrated KCl solution. The microelectrode is connected to the voltage amplifier of the recording device. The membrane potential of muscle cells, nerve cells, or other tissue cells can be measured. Another electrode (reference) is installed on the surface of the tissue.
When the microelectrode tip is outside the cell, its potential relative to the reference electrode is zero. If the end of the electrode is immersed in the cell, piercing the plasma membrane, the potential difference suddenly becomes negative. The potential difference between the internal and external environments of the cell is recorded on the scale of the measuring device. This potential difference is called transmembrane, or membrane potential.
If a cell is at rest, its membrane potential is negative meaning and a stable value. It is usually called resting membrane potential
. The resting membrane potential of cells of various tissues ranges from -
55 millivolts (mV) before -
100mV.
Under certain physiological conditions, changes in membrane potential can occur. Changes in a positive direction are called depolarization
plasma membrane. A shift in membrane potential in a negative direction is called hyperpolarization
.
Biophysical basis of resting membrane potential
Electrical phenomena in the plasma membrane are determined by the distribution of ions between the inner and external sides membranes. From chemical analysis it is known that the concentration of ions in the intracellular fluid is very different from the concentration of ions in the extracellular fluid. The term "extracellular fluid" refers to all fluids outside of cells (intercellular substance, blood, lymph, etc.). The table shows the concentrations of major ions in mammalian muscle cells and extracellular fluid (millimoles per liter).
There are significant differences between the concentration of major ions inside and outside the cell. Extracellular fluid has a high concentration of sodium and chlorine ions. The intracellular fluid has a high concentration of potassium and various organic anions (A -) (charged protein groups).
The difference between the concentrations of sodium and potassium in extracellular and intracellular fluids is due to the activity of the sodium-potassium pump, which pumps 3 sodium ions out of the cell in one cycle and pumps 2 potassium ions into the cell against the electrochemical gradient of these ions. The main function of the sodium-potassium pump is to maintain differences in the concentrations of sodium and potassium ions on both sides of the plasma membrane.
At rest, the permeability of the plasma membrane for potassium ions significantly exceeds the permeability of the membrane for sodium ions. IN nerve cells the permeability ratio of the corresponding ions is 1:0.04.
This fact makes it possible to explain the existence of the resting membrane potential.
Potassium ions tend to leave the cell due to their high internal concentration. In this case, intracellular anions do not move through the membrane due to their large size. The insignificant entry of sodium ions into the cell also does not compensate for the release of potassium ions outside, since the permeability of the membrane at rest for sodium ions is small.
Consequently, the outside of the cell acquires an additional positive charge and an excess of negative charge remains inside.
Potassium diffusion through the membrane is a limited process. Potassium ions passing through the membrane create an electric field that retards the diffusion of other potassium ions. As potassium leaves the cell, the electric field increases and, ultimately, the tension reaches such a value that the flow of potassium through the membrane stops. The state in which the flow of ions along their concentration gradient is balanced by the membrane potential is called state of electrochemical equilibrium ions. The magnitude of this membrane equilibrium potential is determined Nernst equation ( it is believed that the membrane is permeable to only one type of ion ) :
R- universal gas constant, T- thermodynamic temperature, z- electric charge of the ion, F- Faraday constant, i and o - intracellular and extracellular concentrations of potassium ions, respectively.
Calculations based on the Nernst equation indicate that the internal and external concentration of chlorine ion also corresponds to a state of electrochemical equilibrium, but the sodium concentration is far from equilibrium with the membrane potential.
The Nernst equation shows that the concentration gradient of potassium ions determines the value of the resting membrane potential only in a first approximation. The calculated values of the membrane potential coincide with those obtained experimentally only at high potassium concentrations outside the cell.
A more accurate value for the resting membrane potential can be calculated from the Goldman-Hodgkin equation, which takes into account the concentrations and membrane permeability of the three main ions in intra- and extracellular fluids:
Also, the sodium-potassium pump directly participates in maintaining the resting membrane potential, pumping three sodium ions out of the cell and pumping only two potassium ions. As a result, the resting membrane potential becomes more negative than it would be if it were generated solely by the passive movement of ions across the membrane.
Action potential
If a short-term electrical current passes through the membrane of a nerve or muscle cell, the membrane potential undergoes successive changes that are specific and unique to excitable cells. Excitable tissues can also be stimulated by mechanical or chemical means, but in experimental work Electrical stimuli are typically used.
 Rice. 1. Action potential of a nerve cell.
Rice. 1. Action potential of a nerve cell.
Action potential - rapid fluctuation of the membrane potential caused by the action on excitable cell electrical or other stimuli.
In Fig. Figure 1 shows the action potential of a nerve cell recorded using a microelectrode. If a brief electrical stimulus is applied to a cell, the membrane potential decreases rapidly to zero. This deviation is characterized as depolarization phase
And. Within a short time, the internal environment of the cell becomes electropositive relative to the external environment ( membrane potential reversal phase, or overshoot
). The membrane potential then returns to the level of the resting membrane potential ( repolarization stage
) (Fig. 2.).
 Rice. 2. Action potential phases
Rice. 2. Action potential phases
Action potential duration ranges from 0.5 to 1 millisecond in large nerve cells and several milliseconds in skeletal muscle cells. Total amplitude - almost 100 - 120 mV, deviation from the zero line is about 30-50 mV.
The action potential plays a leading role in information processing in the nervous system. It has a constant amplitude, which is not a probabilistic quantity. It has great importance in information processing by the nervous system. The intensity of stimulation is encoded by the number of action potentials and the frequency with which action potentials follow each other.
Biophysical basis of action potential
Action potentials arise from specific changes in ion permeability in the plasma membrane. The English physiologist Hodgkin showed that the main mechanism of the action potential is a short-term and very specific change in the permeability of the membrane to sodium ions. In this case, sodium ions enter the cell until the membrane potential reaches the electrochemical equilibrium potential of sodium ions.
 Rice. 3. Changes in membrane permeability to sodium and potassium ions during an action potential
Rice. 3. Changes in membrane permeability to sodium and potassium ions during an action potential
The permeability of the membrane to sodium when an electrical stimulus is applied to the cell increases approximately 500 times and becomes significantly greater than the permeability of the membrane to potassium ions. The concentration of sodium ions in the cell increases sharply. As a result, the membrane potential takes on a positive value, and the flow of sodium ions into the cell slows down.
During the occurrence of an action potential, depolarization of the plasma membrane occurs. Rapid depolarization of the membrane under the influence of an electrical stimulus causes an increase in its permeability to sodium ions. The increased intake of sodium ions into the cell increases the depolarization of the membrane, which, in turn, causes a further increase in the permeability of the membrane to sodium, etc.
But the value of the membrane potential during depolarization does not reach the level of the electrochemical equilibrium potential of sodium ions. The reason for this is a decrease in the permeability of the membrane to sodium ions due to inactivation of sodium transmembrane transport. This process sharply reduces the permeability of the membrane to sodium ions and stops the influx of sodium into the cell.
At this moment, the membrane permeability to potassium ions increases, which leads to a rapid decrease in the membrane potential to the resting potential level. The permeability of the membrane to potassium ions also decreases to its normal value. Thus, inactivation of the incoming sodium current and increasing the permeability of the membrane to potassium ions (outgoing current) limit the duration of the action potential and lead to repolarization membranes.
Thus, during an action potential, some sodium ions enter the cell. But this amount is quite small. The change in ion concentration in large nerve cells is only about 1/300,000 of the initial value.
The main mechanism of changes in membrane permeability is due to events in the sodium and potassium channels of the membrane. The state of their gates is controlled by the magnitude of the membrane potential. Sodium channels have two types of gates. One of them, called the activation gate, is closed at rest and opens when the membrane is depolarized. The entry of sodium ions into the cell causes the opening of everything more activation gate. The second type of gate of sodium channels - inactivated by increasing depolarization, the membranes gradually close, which stops the flow of sodium into the cell. Depolarization of the membrane also causes the opening of an additional number of potassium channels, as a result of which the permeability of the membrane for potassium ions increases and membrane repolarization occurs.
 Rice. 4. Changes in the state of sodium and potassium channels of the membrane depending on the value of the membrane potential
Rice. 4. Changes in the state of sodium and potassium channels of the membrane depending on the value of the membrane potential
Propagation of action potential
The action potential propagates along the membrane of nerve and muscle cells without decreasing in amplitude with distance. This process is due cable properties plasma membrane, i.e. ability to conduct electric current over short distances. Local electrical current flows into the cell in the active region (where the action potential occurs) and out of the cell in the adjacent inactive region. These ionic currents cause some changes in the membrane potential in the area adjacent to the site of the action potential.
A cyclic local current reduces the membrane charge in the inactive zone and depolarizes it. If depolarization reaches a threshold level, the permeability of the membrane to sodium ions increases and an action potential occurs. Thus, the action potential propagates along nerve and muscle fibers at a constant speed.
 Rice. 5. Action potential propagation along the nerve fiber membrane
Rice. 5. Action potential propagation along the nerve fiber membrane
The speed of action potential propagation in nerve fibers depends on their diameter. It is maximum in the thickest fibers, reaching about 100 meters per second.
One of the most important functions of a biological membrane is the generation and transmission of biopotentials. This phenomenon underlies the excitability of cells, the regulation of intracellular processes, the functioning of the nervous system, the regulation of muscle contraction, and reception. In medicine, diagnostic methods are based on the study of electric fields created by the biopotentials of organs and tissues: electrocardiography, electroencephalography, electromyography and others. Therapeutic effects on tissues and organs with external electrical impulses during electrical stimulation are also practiced.
During life, electrical potential differences may arise in cells and tissues: Δj
1) redox potentials - due to the transfer of electrons from one molecules to others;
2) membrane - due to the ion concentration gradient and the transfer of ions through the membrane.
Biopotentials recorded in the body are mainly membrane potentials.
Membrane potential called the potential difference between the inner (cytoplasmic) and outer surfaces of the membrane:
j m = j out - j int.(1)
Progress in the study of biopotentials is due to:
1) development of a microelectrode method for intracellular potential measurement;
2) the creation of special biopotential amplifiers (UPB);
3) selection of successful objects for studying large cells and among them giant squid axon. The diameter of the squid axon reaches 0.5 mm, which is 100 - 1000 more than the diameter of the axons of vertebrates, including humans. The gigantic size of the axon is of great physiological importance - it ensures rapid transmission nerve impulse along the nerve fiber.
For biophysics, the giant squid axon has served as an excellent model object for studying biopotentials. A microelectrode can be inserted into a squid giant axon without causing significant damage to the axon.
A glass microelectrode is a glass micropipette with a very thin tip (Fig. 5.1 ).
A metal electrode of this thickness is ductile and cannot puncture cell membrane, in addition, it is polarized. To avoid electrode polarization, non-polarizing electrodes are used, such as silver wire coated with salt AgCl In solution KS1 or NaCl(gelatinized agar-agar) filling the microelectrode.
The second electrode, the reference electrode, is located in the solution near the outer surface of the cell. Recording device P containing an amplifier direct current, measures the membrane potential:
Fig.5.1 - Microelectrode method for measuring biopotentials
a - glass micropipette; b - glass microelectrode;
c - scheme for recording membrane potential
The microelectrode method made it possible to measure biopotentials not only on the giant squid axon, but also on normal-sized cells: nerve fibers of other animals, skeletal muscle cells, myocardial cells and others.
Membrane potentials are divided into resting potentials and action potentials.
Resting potential- stationary electrical potential difference recorded between the inner and outer surfaces of the membrane in an unexcited state.
The resting potential is determined by the different concentrations of ions on different sides of the membrane and the diffusion of ions across the membrane.
If the concentration of any ion inside the cell C ext is different from the concentration of this ion outside C nar and the membrane is permeable to this ion, a flow of charged particles occurs through the membrane, as a result of which the electrical neutrality of the system is disrupted, a potential difference is formed inside and outside the cell j m = j out - j out which will prevent further movement of ions through the membrane. When equilibrium is established, the values of electrochemical potentials on opposite sides of the membrane are equalized: m in = m in .
Because m = m 0 + RTlnC + ZFj, That
RTlnC vn + ZFj vn = RTlnC nar + ZFj nar
It's easy to get from here Nernst formula for equilibrium membrane potential
j m = j nar - j int = - RT/ZF´ln(C int / From nar)
If the membrane potential is due to the transfer of K + ions, for which [K + ] in > [K + ] out and Z = +1, the equilibrium membrane potential
For Na + ions: ext< нар, Z = +1,
If in the Nernst formula we move from the natural logarithm to the decimal one, then for a positive monovalent ion (Z = +1)
Let's take the temperature T=300 K, then
Let us accept in the Nernst formula C in / C nar ≈100, which in order of magnitude corresponds to the experimental data for potassium:
log, and membrane potential
0.06∙2V = 0.12V = 120mV,
which is slightly greater than the modulus of the experimentally measured values of the resting potential, and, using the formulas of electrostatics, we will estimate how many ions must move from the cytoplasm to the noncellular environment in order to create such a potential difference. Cell radius r = 10 μm = 10 -5 m. Specific electrical capacitance of the membrane (electrical capacity per unit area) C beat = 10 -2 F/m 2. Membrane area 4πr 2 ≈ 4π∙10 -10 m 2 ≈10 -9 m 2. Then the electrical capacity of the membrane
C=C beat ∙S≈10 -2 ∙10 -9 m 2.
The absolute value of the charge of each sign on the surface of the membrane, if we imagine it as a capacitor,
which corresponds to
Cell volume
The change in ion concentration in the cell due to the release of 10 -17 mol ions from the cell will be
The small change in concentration compared to the change in the concentration of potassium ions inside the cell is only 10 -4% of the potassium concentration inside the cell. Thus, in order to create an equilibrium Nernst membrane potential, a negligibly small number of ions must pass through the membrane compared to the total number in the cell.
Thus, the resting potential is actually closer to the potential calculated using the Nernst formula for K +. At the same time, a significant discrepancy between the experimental and theoretical values is noteworthy. The reason for the discrepancy is that the permeability of the membrane for other ions is not taken into account. The simultaneous diffusion of K +, Na + and C1 - ions through the membrane is taken into account by the Goldmann equation.
The Goldmann equation can be derived from the Nernst-Planck equation.
Let's transform this equation:
URT=D according to Einstein's relation. Let us accept the so-called Goldmann constant field approximation. We will consider the electric field strength in the membrane constant and equal to the average value of the potential gradient:
Where l– membrane thickness.
We obtain for the density of ion flux through the membrane:
We denote We write
Let's separate the variables:
Let's integrate the left side of the differential equation in the range from 0 to 1, and the right side from C nar = KS nar to C in = KS in (where K is the distribution coefficient)
After potentiation
Let's express it from here:
Considering that , we get:
In the stationary case, when the potential difference - the membrane potential - inhibits further transfer of ions through the membrane, the total flux of various ions becomes equal to zero:
j K + + j Na + - j Cl - = 0
Before j there is a minus sign, taking into account the negative charge of the chlorine ion. However, since various ions participate in creating the membrane potential, equilibrium does not occur; the fluxes of various ions are not equal to zero individually. If we take into account only the flows j K + And jNa+, That j K+ +j Na+ =0, or j K = - j Na + and, substituting, we get:
Because the,
If we also take into account the ion flow C1 -, then, repeating the previous arguments, we can obtain an equation for the membrane potential created by the flows through the membrane of three types of ions, Goldmann equation:
The numerator of the expression under the logarithm sign represents the concentrations [K + ] VN, BH, but [C1 - ] NAR, and in the denominator - [K + ] NAR, H AR, But [C1 - ] VN, since chlorine ions are negatively charged.
At rest, the permeability of the membrane for K + ions is significantly greater than for Na +, and greater than for C1 -:
P K >>P Na , P K >P Na .
For a squid axon, for example,
P K:P Na:P Cl =1:0.04:0.45.
Rewriting the Goldman equation as:
in the case when the permeability of the membrane for sodium and chlorine ions is significantly less than the permeability for potassium:
P Na<< P K , P Cl << P K ,
Thus, the Nernst equation is a special case of the Goldmann equation.
The membrane potential calculated using the Goldman equation turned out to be less in absolute value than the membrane potential calculated using the Nernst formula, closer to its experimental values in large cells. Both the Nernst formula and the Goldman equation do not take into account the active transport of ions through the membrane, the presence in the membranes of electrogenic (causing separation of charges, and therefore the occurrence of potential differences) ion pumps, which play an important role in maintaining ionic balance in small cells. K + -Na + -ATPases work in the cytoplasmic membrane, pumping potassium into the cell and sodium out of the cell. Taking into account the operation of electrogenic ion pumps for the membrane potential, it was obtained Thomas equation:
where m is the ratio of the number of sodium ions to the number of potassium ions pumped by ion pumps through the membrane. Most often, the K + -Na + -ATPase operates in the mode when m = 3/2, m is always greater than 1. (There are no ion pumps pumping Cl, therefore, in the Thomas equation there are no terms P Cl [Cl -].)
The coefficient m > 1 enhances the contribution of the potassium concentration gradient to the creation of the membrane potential, so the membrane potential calculated by Thomas is greater in absolute value than the membrane potential calculated by Holman and agrees with experimental values for small cells.
Disruption of bioenergetic processes in the cell and the work of K + -Na + -ATPase leads to a decrease in |φ m |, in this case the membrane potential is better described by the Goldmann equation.
Damage to the cell membrane leads to an increase in the permeability of cell membranes for all ions: to an increase in Pk, PNa, and Pcl. Due to a decrease in the difference in permeability, the absolute value of the membrane potential |φ m | decreases.
For severely damaged cells |φ m | even less, but the negative membrane potential |φ m | due to the polyanions contained in the cell - negatively charged proteins, nucleic acids and other large molecules that cannot penetrate the membrane (Donnan potential).
Action potential
Through electrical nerve impulses (action potentials) in a living organism, information is transmitted from receptors to brain neurons and from brain neurons to muscles. A living organism is a completely electrified system. Without electricity there is no life.
The action potential was opened before the resting potential. Animal electricity has been known for a long time. Electric eel discharges (occurring at voltages up to 600 V, with a current of about 60 A and a duration of about a millisecond) were used by medicine back in Ancient Rome to treat gout, headaches, and epilepsy. The electrical nerve impulse was discovered by Luigi Galvani, professor of anatomy in Bologna. The results of his electrophysiological experiments are presented in the book “Treatise on the Forces of Electricity in Muscular Movement” (1791). Galvani discovered that muscle contractions of the limbs of a dissected frog could be caused by an electrical impulse and that the living system itself was the source of the electrical impulse. Galvani's great discovery played an outstanding role in the development of physics, electrical engineering, electrochemistry, physiology, biophysics and medicine. However, the enormous popularity of Galvani's ideas led to their profanation, traces of which have remained to this day (galvanization of corpses, galvanism of eye contact, etc.), which caused distrust of Galvani's experiments among physicists. Galvani's younger contemporary, physics professor Alessandro Volta, was a fierce opponent of the idea of animal electricity (with the exception of the special cases of electric fish: the electric eel and the electric stingray). In his experiments, he excluded a biological object and showed that an electric current could be generated by the contact of a set of metals separated by an electrolyte (voltage column). This is how a chemical current source was discovered (named, however, later, in honor of its scientific opponent, a galvanic element).
In the 19th century, a primitive idea of the propagation of electrical currents through nerves, as if through wires, was established. However, Helmholtz (second half of the 19th century) showed that the speed of propagation of a nerve impulse is only 1-100 m/s, which is significantly less than the speed of propagation of an electrical impulse through wires up to 3 10 8 m/s. Therefore, by the end of the 19th century, the hypothesis of the electrical nature of the nerve impulse was rejected by most physiologists. It was suggested that a chemical reaction spread along the nerve fibers. In fact, as was later shown, the slow propagation of the electrical nerve impulse is associated with the slow recharging of capacitors, which are cell membranes, through large resistances. The membrane recharging time constant τ= RC is large, since the membrane capacitance (C) and the resistance R of the nerve fiber are large.
The fact that a nerve impulse is an electric current impulse was proven only by the middle of the 20th century, mainly in the works of the English physiologist A. Hodgkin and his colleagues. In 1963, Hodgkin, Huxley and Eakeles were awarded the Nobel Prize in Medicine "for the operation of nerve cells."
Action potential (AP) is an electrical impulse caused by a change in the ionic permeability of the membrane and associated with the propagation of an excitation wave through the nerves and muscles.
Experiments to study the action potential were carried out (mainly by Hodgkin and his colleagues) on squid giant axons using the microelectrode method using high-resistance voltage meters, as well as the labeled atom method. The diagram of experiments and research results are shown in Fig.
In experiments to study the action potential, two microelectrodes inserted into the axon were used. A pulse with amplitude V is supplied to the first microelectrode from a rectangular pulse generator G, which changes the membrane potential. The membrane potential is measured using a second microelectrode by a high-resistance voltage recorder P.
Fig.5.2 - Study of action potential:
a - experimental diagram (G - pulse generator, P - voltage recorder); b - action potential (φ p m - resting potential, φ rev m - reversal potential, φ d m - action potential amplitude, φ po m - threshold potential)
The excitatory impulse causes only a short-term shift in the membrane potential, which quickly disappears and the resting potential is restored. In the case when the excitatory impulse shifts even further in the negative direction, it is accompanied by hyperpolarization of the membrane. Also, an action potential is not formed when the excitatory impulse is positive (depolarizing), but its amplitude is less than the threshold value V nop. However, if the amplitude of the positive, depolarizing pulse turns out to be greater than the value V nop, φ m becomes greater than φ po m and a process develops in the membrane, as a result of which a sharp increase in the membrane potential occurs and the membrane potential φ m even changes its sign - it becomes positive (φ in >φ nar).
Having reached a certain positive value φ rev - reversion potential, the membrane potential returns to the value of the resting potential φ p m, performing something like a damped oscillation. In nerve fibers and skeletal muscles, the duration of the action potential is about 1 ms (and in the cardiac muscle about 300 ms. After the excitation is removed, some residual phenomena are observed in the membrane for another 1-3 ms, during which the membrane is refractory (unexcitable).
A new depolarizing potential V > V nop can cause the formation of a new action potential only after the membrane has completely returned to its resting state. Moreover, the amplitude of the action potential
does not depend on the amplitude of the depolarizing potential (unless V > V nop). If at rest the membrane is polarized (the potential of the cytoplasm is negative in relation to the extracellular environment), then upon excitation the membrane depolarizes (the potential inside the cell is positive) and after the excitation is removed, repolarization of the membrane occurs.
Characteristic properties of an action potential:
1) the presence of a threshold value of the depolarizing potential;
2) the “all or nothing” law, that is, if the depolarizing potential is greater than the threshold, an action potential develops, the amplitude of which does not depend on the amplitude of the exciting impulse and there is no action potential if the amplitude of the depolarizing potential is less than the threshold;
3) there is a period of refractoriness, non-excitability of the membrane during the development of the action potential and residual effects after removal of excitation;
4) at the moment of excitation, the membrane resistance sharply decreases (in the squid axon from 0.1 Ohm m 2 at rest to 0.0025 Ohm m 2 during excitation).
If we turn to the data for the values of equilibrium Nernst potentials created by various ions, it is natural to assume that the positive reversion potential is of sodium nature, since it is the diffusion of sodium that creates a positive potential difference between the inner and outer surfaces of the membrane.
You can change the amplitude of the action potential pulse by changing the sodium concentration in the external environment. As the external sodium concentration decreases, the amplitude of the action potential decreases as the reversal potential changes. If sodium is completely removed from the environment surrounding the cell, an action potential does not arise at all.
Experiments carried out with a radioactive isotope of sodium made it possible to establish that upon excitation, the permeability to sodium increases sharply. If, at rest, the ratio of the permeability coefficients of the squid axon membrane for different ions is:
P K: P Na: P Cl = 1: 0.04: 0.45
then in a state of excitement:
P K: P Na: P Cl = 1: 20: 0.45
that is, compared to the unexcited state, upon excitation the permeability coefficient for sodium increases 500 times.
Calculations of the membrane reversion potential using the Goldmann equation, if we substitute the membrane permeability values for the excited state into it, coincide with the experimental data.
Excitation of the membrane is described by the Hodgkin-Huxley equations. One of the Hodgkin-Huxley equations has the form:
where I m is the current through the membrane, C m is the membrane capacitance, ∑I i is the sum of ionic currents through the membrane.
Electricity through the membrane consists of ionic currents: potassium ions - I k +, sodium - I Na + and other ions, including Cl, the so-called leakage current I k, as well as capacitive current. The capacitive current is caused by the recharging of the capacitor, which is a membrane, by the flow of charges from one surface to another. Its value is determined by the amount of charge flowing from one plate to another per unit time dq/dt, and since the charge of the capacitor is q = C m ∆φ = C m φ m, then the capacitive current is C M. Total membrane current
According to the Hodgkin-Huxley theory, excitation of a membrane element is associated with changes in membrane conductivity for Na + and K + ions: g K and g Na.
Membrane conductances depend in complex ways on membrane potential and time.
It was found that if the membrane potential is raised (φ m above the threshold), current first flows into the cell, and then out of the cell.
In experiments conducted by Hodgkin, Huxley, Baker, Shaw, it was proven that phase I of the membrane current is associated with the flow of sodium ions from environment(where the sodium concentration is greater) into the cell (where it is less), and phase II is explained by the flow of potassium ions out of the cell.
In their experiments, Hodgkin and Huxley changed the ionic composition of the surrounding solution. It was found that if sodium was removed from the outside, the first phase of the membrane current (current into the cell) disappeared. Therefore, in fact, the first phase of the development of the action potential is associated with an increase in the permeability of the membrane to sodium ions. The flow of positive particles into the cell leads to depolarization of the membrane - its inner surface is charged positively in relation to the outer one.
In the second phase, the permeability of the membrane to potassium sharply increases and positively charged potassium ions leave the cell, while the sodium current decreases. The ionic mechanism of action potential development was finally proven in the decisive experiment of Hodgkin, Baker and Shaw, in which the axoplasm of a dissected axon was replaced by an external solution, and the ionic composition of the external solution was made the same as that of normal axoplasm. With such a replacement of ionic compositions, the potential difference on the membrane changed sign. Now, at rest, its inner surface was charged positively in relation to the outer one. And the action potential turned out to be negative.
It has been hypothesized that the selective (selective) change in the ionic permeability of the excited membrane: first for Na +, and then for K + - is explained by the fact that there are special ion channels in the membrane. There are separate sodium and potassium channels that open and close as a nerve impulse passes through a given area of the membrane. In the first phase, sodium channels open, in the second phase, potassium channels open. Accordingly, sodium channels close first, and then potassium channels. The opening and closing of ion channels is caused by changes in membrane potential.
One of the evidence for the presence of ion channels in the membrane is the existence of substances that block ion flows through the membrane. Thus, tetrodotoxin contained in fugu fish blocks the entry of sodium into the cell and, thus, disrupts the transmission of nerve impulses, which can lead to death. It has been proven that tetrodotoxin does not affect the permeability of cells to potassium, which means that sodium and potassium ions actually pass through different channels. Due to its specific structure, tetrodotoxin molecules appear to get stuck in sodium channels. By counting the number of tetrodotoxin molecules stuck in the membrane, it was possible to determine the number of sodium channels. In different nerve fibers of vertebrates it was different - from 3 to 75 channels per square micrometer of membrane area (for comparison, the number of phospholipid molecules is ≈ 2 10 6 1 / μm 2).
A specific inhibitor of potassium channels was also discovered - tetraethylammonium. If the membrane is treated with tetrodotoxin, which blocks sodium channels, the first phase disappears in experiments with fixation of the membrane potential, and tetraethylammonium, which stops the transfer of potassium through the membrane, causes the disappearance of the second phase.
Thus, it has been established that the formation of an action potential is caused by ionic flows through the membrane: first, sodium ions into the cell, and then potassium ions from the cell into the external solution, which is associated with a change in the conductivity of the membrane for potassium and sodium ions.